1 Background
1.1 Pharmac’s Role and Functions in the New Zealand Health System
Pharmac, the Pharmaceutical Management Agency, is a Crown entity that is directly accountable to the Minister of Health. Our functions are set out in section 48 of the New Zealand Public Health and Disability Act 2000 (NZPHD Act).
One of Pharmac’s functions is to manage the Pharmaceutical Schedule (the Schedule), which is the list of pharmaceuticals that are publicly funded. Funding may be for either or both the community or hospital setting. The definition of ‘pharmaceuticals’ is broad: as well as medicines, it includes vaccines, medical devices, and related products and things(1).
Pharmac’s statutory objective is:
to secure for eligible people in need of pharmaceuticals, the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided. (1, Section 47(a)(external link))
Further information on Pharmac can be found at www.pharmac.govt.nz.
1.2 Purpose of the Prescription for Pharmacoeconomic Analysis
This document is intended as guidance for use by anyone preparing economic analyses for PHARMAC, including PHARMAC staff, pharmaceutical companies, and other health economists. It provides guidance on methods for any economic assessment that will help to inform a PHARMAC funding decision.
Assessment of a proposal against the Factors for Consideration is supported by the use of economic analyses as described in this document. PHARMAC’s preferred method is ‘cost-utility analysis’ as defined in this document; although cost-minimisation analyses may be sufficient for some proposals.
As all proposals are considered and prioritised against all other possible uses of available funds at any time, it is important that common methods are used for all assessments wherever possible. A consistent approach to economic analyses as recommended in this document will help ensure proposals can be fairly compared with one another.
The focus of cost-utility analysis is on benefits as formally measured by Health-Related Quality of Life (Chapter 6) and on costs as defined in Chapter 7. Many other aspects are important to any funding decision. PHARMAC’s Factors for Consideration provide a framework to ensure that all relevant aspects and issues are taken into account. The economic assessments described in the Prescription for Pharmacoeconomic Analysis (PFPA) can help inform consideration of many but not all of the Factors.
The PFPA does not in any way attempt to be a comprehensive academic document or to describe all the technical details of cost-utility analysis. It also does not attempt to provide a thorough description of PHARMAC’s prioritisation process or to provide detailed guidance for assessing technologies that are beyond PHARMAC’s scope. Rather, it describes the process involved and methods used when conducting health economic analyses, in particular cost-utility analyses. It includes specific guidance on how PHARMAC measures costs (Chapter 7) and benefits (Chapter 6), and how it allows for time preference (Chapter 8 and Appendix 2) and uncertainty (Chapter 10).
1.3 History of the PFPA
The idea of standardising and documenting the methods PHARMAC uses for economic analyses started in 1997. At that time, PHARMAC had undertaken a number of cost-utility analyses and considered it would be useful to formalise and standardise its approach.
PHARMAC consulted widely on the draft manual, and comments were received from leading national and international health economists, clinicians, the pharmaceutical industry, and the then Health Funding Authority. Following amendments to the draft version, the manual was finalised and published on the PHARMAC website as the Prescription for Pharmacoeconomic Analysis in September 1999.
In 2004, PHARMAC decided to review and revise the PFPA. A literature search was undertaken and internal sessions were held to review each section. The draft new version of the PFPA was subsequently reviewed by international and New Zealand experts in cost-utility analysis. PHARMAC staff then consulted widely on the new draft, considered all consultation responses, and as a result made a number of amendments to the document. Version 2 of the PFPA was approved by the PHARMAC Board in April 2007 and published in June 2007.
Version 2.1 was published in 2012. The changes from version 2.0 were various technical amendments and clarifications to reflect PHARMAC’s current practice.
1.4 PFPA Version 2.2
This update of the PFPA supports the introduction of Factors for Consideration and changes to PHARMAC’s role.
PHARMAC’s scope in the health system has developed over time. In 2012/13, PHARMAC was given responsibility for the National Immunisation Schedule, which has now been incorporated in to the Pharmaceutical Schedule. In 2013/14, PHARMAC added hospital medicines and also listed national contracts for hospital medical devices.
Version 2.2 of the PFPA specifically takes account of responses to consultation on the expansion of PHARMAC’s scope to products including hospital medical devices, vaccines, and some haemophilia products.
1.5 Decision making and the PFPA
This version 2.2 of the PFPA gives guidance for analyses that support PHARMAC decisions that take account of the Factors for Consideration. Analyses intended to take account of the earlier nine Decision Criteria should be guided by version 2.1 of the PFPA. Detailed information on the move from Decision Criteria to Factors for Consideration can be found at
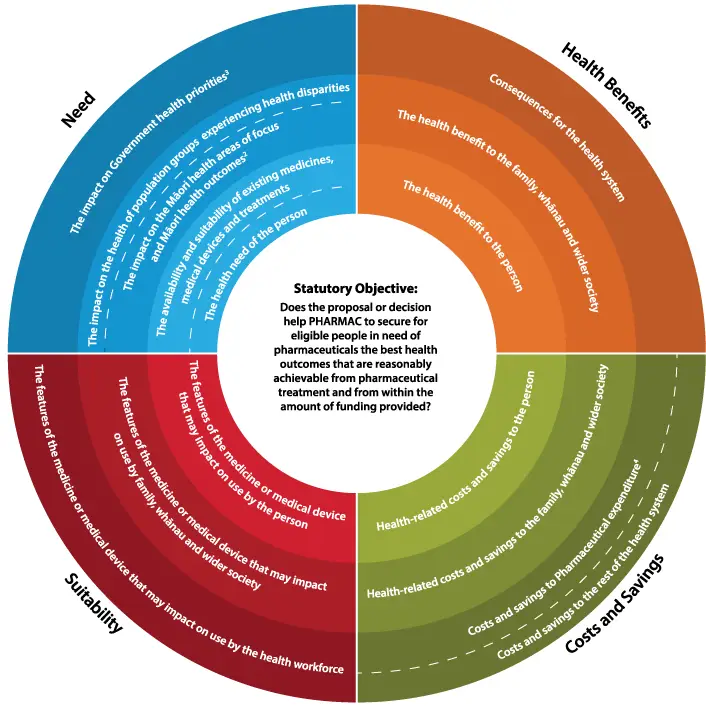
The Factors for Consideration are shown in the following diagram. The circular diagram represents the four different dimensions/quadrants that PHARMAC will generally consider when making funding decisions (Need[1], Health Benefits, Costs and Savings, and Suitability), and the three levels of impact that we will usually take into account (to the person; to the person’s family, whānau and wider society; and to the broader health system). Ultimately, these Factors help to ensure we meet our Statutory Objective: “to secure for eligible people in need of pharmaceuticals, the best health outcomes that are reasonably achievable from pharmaceutical treatment and from within the amount of funding provided”.
Cost-utility analysis as described in the PFPA mainly helps inform the Health Benefits and the Cost and Savings dimensions. An assessment may also generate information helpful for considering Factors within the Need and Suitability dimensions.