Exceptional Circumstances Framework
Legislative function
Pharmac’s role includes considering whether to fund pharmaceutical treatments for people in exceptional circumstances when those treatments are not currently available for them on the Pharmaceutical Schedule.
This role reflects the legislative function outlined in section 69(1)(b) of the Pae Ora (Healty Futures) Act 2022:
[Managing] incidental matters arising out of [maintaining and managing a pharmaceutical schedule], including in exceptional circumstances providing for subsidies for the supply of pharmaceuticals not on the pharmaceutical schedule.
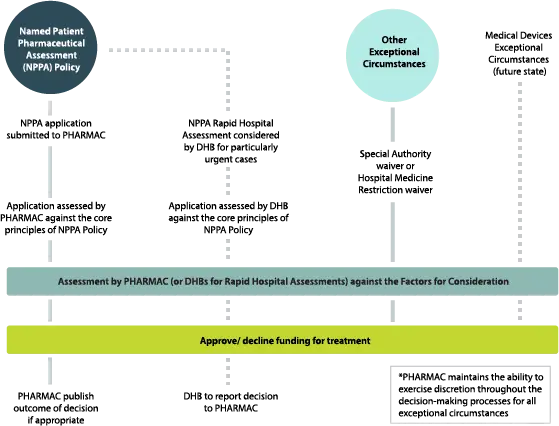
The Exceptional Circumstances Framework outlines the ways in which Pharmac generally considers funding decisions for exceptional circumstances that fall outside of the Pharmaceutical Schedule funding process, and guides Pharmac’s decision making in these cases. The Framework includes the Named Patient Pharmaceutical Assessment (NPPA) Policy and other processes through which Pharmac considers exceptional circumstances. In the future this will also include an exceptional circumstances policy or process for Medical Devices.
The Exceptional Circumstances Framework does not limit Pharmac's ability to consider any application for funding treatments outside of the NPPA Policy or any other exceptional circumstances processes, at its discretion.
Section 1: Named Patient Pharmaceutical Assessment Policy
Pharmac developed the Named Patient Pharmaceutical Assessment (NPPA) Policy in order to guide it as it carries out its legislative function to consider funding for pharmaceutical treatments in exceptional circumstances.[1] The Policy sets out some core principles which applications will be considered against by Pharmac (or DHBs in the case of NPPA Rapid Hospital Assessments), to determine if a treatment should be considered for funding. If the application is consistent with the core principles of the NPPA Policy, the application will then be assessed against the Factors for Consideration.
In considering an application against the core principles of the NPPA Policy, Pharmac has the discretion to determine whether it would be more appropriate to deal with the application through other funding pathways. Pharmac always maintains its discretion to fund treatments for individuals in appropriate circumstances, even if those circumstances do not meet the core principles of the NPPA Policy.
Core principles of the NPPA Policy:
1. The NPPA Policy provides a pathway to consider those whose clinical circumstances cannot be met through the Pharmaceutical Schedule at a given point in time
Pharmac recognises that there are some clinical circumstances for which a given treatment is not available through the Pharmaceutical Schedule, or cannot feasibly be considered through the Pharmaceutical Schedule listing process at a given point in time. The NPPA Policy process in combination with the Pharmaceutical Schedule listing process ensures there is a ‘door’ to consider funding for all patients.
2. The NPPA Policy complements the Pharmaceutical Schedule and the Schedule decision-making process
The NPPA Policy must operate only to consider those funding applications which are not appropriate to be considered through the Pharmaceutical Schedule listing process; for example due to the highly unusual nature of an individual’s clinical circumstances. This ensures that the NPPA Policy does not undermine the Schedule through providing an alternative competing pathway for funding in the same circumstances.
3. The NPPA Policy is designed for individual assessment
While the Pharmaceutical Schedule lists medicines for population groups, the NPPA Policy considers applications to fund treatments for individuals. This acknowledges that some individuals have specific clinical circumstances that may be different to a wider patient population. However, if a person has the same clinical circumstances as a wider patient population, the Pharmaceutical Schedule may be the more appropriate pathway to consider these circumstances so that all people with the same condition have equal opportunity to access the treatment.
Application process for NPPA
Meeting the NPPA Policy core principles
The assessment process for considering the funding of a treatment through the NPPA Policy is comparable to the Schedule listing process, in that the application is assessed against the Factors for Consideration. However, before reaching this stage, Pharmac (or DHBs in the case of Rapid Hospital Assessments) will first consider whether a funding application is consistent with the core principles of the NPPA Policy.
Below are three key questions that should be addressed when determining if an application is consistent with the NPPA Policy core principles. The corresponding boxes below demonstrate how the core principles are relevant to the three key questions guiding the NPPA Policy process.
Clinicians should consider and address questions 1 and 2 when making their NPPA application, and Pharmac (or DHBs in the case of Rapid Hospital Assessments) will consider all three questions when assessing a NPPA application.
Pharmac may use its discretion to consider an application under the Factors for Consideration in circumstances where the NPPA Policy core principles are not met but there is justifiable reason for the application to be considered through NPPA. Circumstances may include where a pharmaceutical is less expensive than the funded alternative.[3]
| If an application is consistent with the core principles of the NPPA Policy then Pharmac (or DHBs in the case of Rapid Hospital Assessments) will assess the application against the Factors for Consideration. In order to assess whether an application is consistent with the core principles, the questions below need to be addressed. | |
| 1. Does the person have exceptional clinical circumstances? | Principle 1: The NPPA Policy is intended to meet the needs of those exceptional cases which are not currently feasible to consider through the Pharmaceutical Schedule listing process. This may be due to the urgent clinical need of the person, or the unusual nature of their particular clinical circumstances. |
| Principle 3: The NPPA Policy is designed for individual assessment but it must be determined how the individual’s clinical circumstances are ‘different’ to the wider patient population, and the implications of funding this individual in the context of the wider patient population must also be considered. | |
| 2. Has the person tried all existing funded alternative treatments? | Principle 1: The Pharmaceutical Schedule does not list all the treatments that might meet the needs of all individual clinical circumstances. However, where suitable funded options are available they must be tried before seeking funding for treatments via the NPPA Policy.[3a] |
| Principle 2: Treatments funded through NPPA must be ‘end of spectrum’ in the sense that other currently funded options are not clinically suitable for the individual. This ensures that the NPPA process does not undermine the Pharmaceutical Schedule through providing a competing alternate pathway to access funded treatment. | |
| 3. Has Pharmac considered the treatment for funding previously? | Principle 2: Treatments funded through NPPA must not have been considered by Pharmac already for the indication which is the subject of the application, through the Pharmaceutical Schedule application process. |
| Principle 3: If a treatment has been considered for funding through the Pharmaceutical Schedule application process but the person’s clinical circumstances are different to those indications which have already been considered by Pharmac, funding the treatment through NPPA may be appropriate. | |
Assessment against the Factors for Consideration[4]
Once applications have been evaluated against the core principles of the NPPA Policy, Pharmac (or DHBs for Rapid Hospital Assessments) will assess the application against the Factors for Consideration as set out in Pharmac's Operating Policies and Procedures.
Pharmac will seek clinical advice when assessing applications against the Factors for Consideration. Clinical advice may be sought from Pharmac’s own clinically qualified staff, members of Pharmac’s clinical advisory panels or committees, or external expert clinical advisors. Where the application relates to clinical circumstances that have previously been assessed by Pharmac, Pharmac will generally use the same clinical advice obtained earlier, unless new clinical information is presented[5].
An important consideration in assessing NPPA applications is determining the benefit that may be forgone from not funding other treatments as a result of funding the treatment in question. Where the cost of a treatment being sought under NPPA is high,[6] more analysis may be required before a decision is made. In such cases, Pharmac may determine that assessment through the Schedule decision-making process is the appropriate pathway for considering funding.
Decisions on NPPA applications
Decisions made under the NPPA Policy relate solely to the named patient who is the subject of the application.
Decisions on NPPA applications submitted to Pharmac will be made by the Pharmac Board or by staff under delegated authority from the Board. Rapid Hospital Assessments undertaken by DHBs must be considered and decided on by a multi-disciplinary panel that consists of at least two individuals, neither of whom are the named patient’s prescriber. DHBs may establish regional panels that consist of staff from multiple DHBs, to consider Rapid Hospital Assessments for all DHBs in that region.
Decisions on applications for treatments that are particularly complex may take more time than decisions on other NPPA applications. This is due to these applications needing more comprehensive analysis.
DHBs that undertake Rapid Hospital Assessments are required to inform Pharmac of the outcome of all Rapid Hospital Assessment decisions, within one month of the decision being made. It is possible that different DHBs may make different Rapid Hospital Assessment decisions on patients with similar clinical circumstances. To reduce variability in outcomes, Pharmac may choose to review a DHB’s Rapid Hospital Assessment decision, and to implement a precedent for future applications of a similar nature.[7] Pharmac may also consider the treatment for Schedule listing.
Information about NPPA decision outcomes
Pharmac will provide information on previous NPPA applications, as appropriate, on the Pharmac website. Published information will include the pharmaceutical funded and the indication it was funded for. Any decisions where the patient may be identifiable will not be published for reasons of privacy. Pharmac may also withhold information for reasons of commercial sensitivity.
We publish a summary table of the outcomes of NPPA applications
Resubmission of an application and decision reviews
Declined applications can be resubmitted at any time if relevant new clinical circumstances arise or new evidence becomes available.
Pharmac has a review process for applicants not satisfied with decisions made under the NPPA Policy. This review process is available for all NPPA applications, including Rapid Hospital Assessments.
Renewal of NPPA funding
Applications approved under the NPPA Policy are for a limited time. If continued funding is required, a renewal application will need to be submitted to Pharmac (or to the DHB where the original application was a DHB-approved Rapid Hospital Assessment). The renewal requires a full clinical update, including evidence of benefit from the treatment.
Schedule decision-making for treatments funded under NPPA
Pharmac may decide to begin a Schedule listing process for a pharmaceutical that is currently (or has been previously) sought for a patient through the NPPA process. Considering the funding of the treatment through the Schedule decision-making process will help ensure that Pharmac considers the benefit of the treatment for all people who might need it.
If a treatment is declined through the Schedule decision-making process by the Pharmac Board, any patient who is receiving that treatment under a current NPPA approval (prior to the Schedule decline decision) will continue to receive funding for the treatment. However, the patient must continue to meet any renewal conditions (discussed in the section above).
Section 2: Other Exceptional Circumstances
Provisions for waivers of criteria or restrictions on Pharmaceutical Schedule listings
Some pharmaceuticals that are listed in the Pharmaceutical Schedule require certain conditions to be met before funding will be granted. These conditions generally ensure that funding is targeted to those patients that would benefit most from treatment. There are circumstances where a person’s clinical circumstances may meet the clear policy intent of the conditions within the Schedule, but the technical requirements are not met. In such circumstances, Pharmac may use its discretion to grant a Special Authority waiver for pharmaceuticals listed in sections B through to D of the Pharmaceutical Schedule including pharmaceutical cancer treatments (PCTs), and vaccines listed in Section I, or a Hospital Medicine Restriction waiver for hospital-dispensed pharmaceuticals (listed in section H of the Pharmaceutical Schedule).
Previous Exceptional Circumstances Schemes
Any individuals receiving funding for treatment under the previous Community Exceptional Circumstances and Cancer Exceptional Circumstances schemes (pre-2012) will continue to receive that funding subject to any renewal conditions.
District Health Boards are responsible for the funding of treatment approved pre-2012 under the Hospital Exceptional Circumstances scheme.
Applications for renewal of funding under the previous schemes will continue to be assessed against the criteria for these schemes. Renewal forms can be accessed on the Pharmac website.
Section 3: Funding of approved treatments under the Exceptional Circumstances framework
For pharmaceuticals supplied in the community and for pharmaceutical cancer treatments (PCTs), funding for NPPA applications approved by Pharmac is provided from within the Combined Pharmaceutical Budget. For approved NPPA applications where the pharmaceuticals are supplied by DHB hospitals (other than PCTs) the funding will be provided from within individual DHB hospital budgets.
In the case of Rapid Hospital Assessments, the DHB that approves the decision is responsible for funding the treatment. If the patient is from another DHB of domicile, or transfers to another DHB, then the approving DHB is required to continue funding the treatment, or to negotiate a funding transfer with the other DHB, if appropriate.
Pharmac is working towards full budget management of hospital pharmaceuticals and there may be future administrative changes to the way the budgets are managed within DHBs. DHBs are not currently able to approve expenditure from the Combined Pharmaceutical Budget.
[1] The NPPA Policy first came into effect 1 March 2012. The policy was revised in March 2013 to include hospital medicines and again following review in 2015. Any changes to the NPPA Policy must be approved by the Pharmac Board.
[2] An authorised prescriber is set out in the Medicines Act 1981 section 2(1).
[3] A funded alternative treatment may be a medicine, device or service that is currently funded in New Zealand. The treatment must also be the funded treatment that most prescribers/clinicians use in New Zealand, and/or the treatment given to the largest number of patients (if this differs from the treatment most prescribers or clinicians use).
[3a] Suitable funded treatments’ acknowledges that some alternative treatments may have been associated with serious side effects or may be contraindicated so are not deemed suitable to have trialled.
[4] This section of the NPPA Policy has been updated now that the Factors for Consideration are in effect.
[5] There will be a written record of previous clinical advice.
[6] Cost of a treatment will be considered for the likely size of the patient group with the exceptional circumstances in question.
[7] An initial positive decision by the DHB would stand, provided the treatment had commenced, regardless of the outcome of a subsequent Pharmac review. However, a negative decision could be overturned should PHARMAC undertake a review and determine that funding is consistent with the NPPA Policy.


