Dipyridamole (Pytazen SR) Tab long-acting 150 mg: Discontinuation
Douglas, the supplier, advises that it can no longer supply this product because of difficulty getting the active ingredient.
12 December 2025 | Update
Dipyridamole has been discontinued in New Zealand. Anyone still taking it will need to move to alternative treatment options.
The Strides brand has been funded temporarily to allow people to do this.
From 1 November 2025, we also widened access to ticagrelor for people who have recently had a minor stroke or high-risk TIA.
Affected product
Douglas, the supplier, advises that it can no longer supply this product because of difficulty getting the active ingredient. We have not been able to find a replacement product.
We're sorry. We know that changing medicines isn't ideal. We have tried to avoid this but were not able to.
Chemical: Dipyridamole
- Presentation: Tab long-acting 150 mg
- Brand: Pytazen SR
- Pharmacode: 750255
- Subsidy: $13.93
- Measure / Qty: per 60
Key dates
May 2024: Douglas advised of the discontinuation
September 2025: Supplier expected Stock of Pytazen SR to run out
1 September 2025: Temporary alternative product funded and available. No new patients restriction added
Later in 2026: Pytazen SR will be delisted
People taking Pytazen SR
You will need to talk to the person who prescribed your Pytazen SR about alternative treatments. We have funded Strides dipyridamole to give you more time to talk to your prescriber.
Strides has the same active ingredient, but a different strength from Pytazen SR. This means you will need a new prescription for the Strides brand.
We're sorry. We know that having to change treatments can be challenging. Despite our best efforts, we have not been able to avoid this discontinuation.
Prescriber action needed
We have not found an alternative to Pytazen SR. You will need to move anyone taking Pytazen SR to an alternative treatment and you can't start anyone on dipyridamole. Our clinical advisors have indicated that, where it is clinically appropriate, prescribers may like to consider:
- clopidogrel as a single agent (especially if a person is allergic to or intolerant of aspirin)
- aspirin as a single agent (especially if a person is resistant to or intolerant of clopidogrel).
- ticagrelor (for people who have recently had a minor stroke or high-risk TIA.)
Our clinical advisers consider aspirin plus clopidogrel in combination should not be used in the long-term secondary prevention of stroke or TIA. It should be used under specialist direction only.
More information
Antiplatelet therapy - MAGICapp(external link) (Australian and New Zealand Living Clinical Guidelines for Stroke Management)
Aspirin monograph | NZ Formulary(external link)
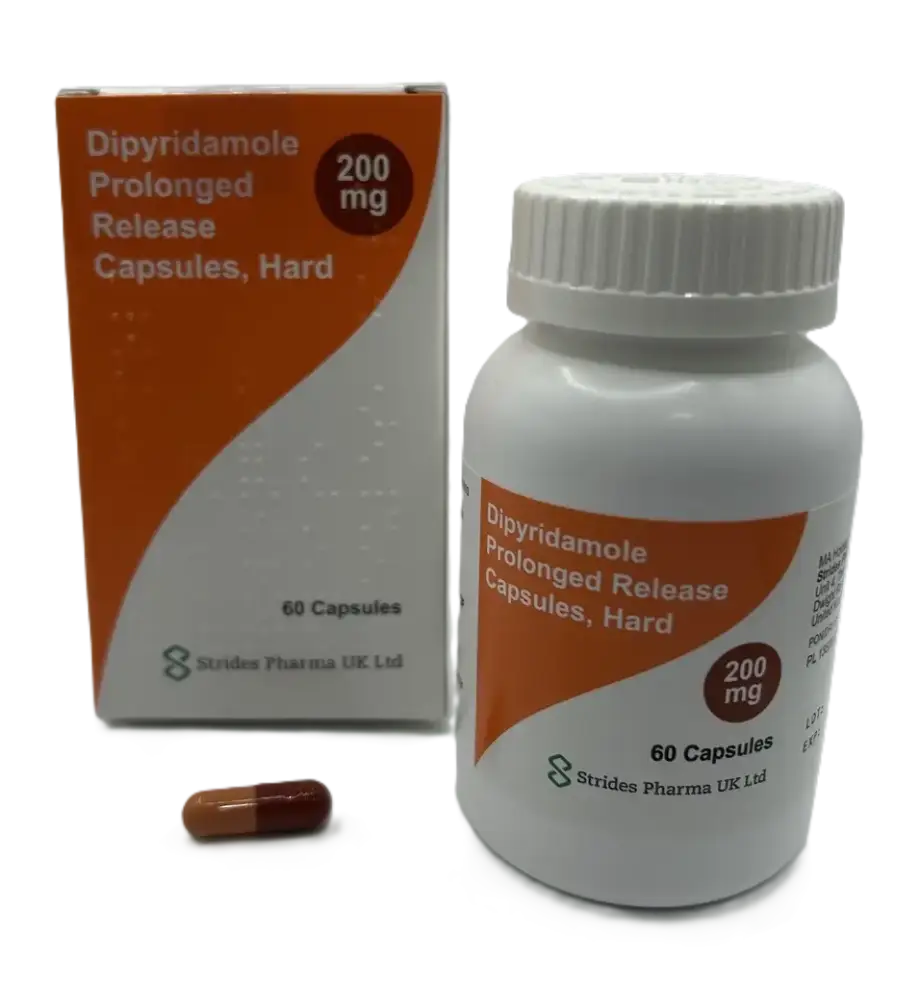
Details of the temporary alternative
This product will be listed from 1 September 2025. It will be supplied by CDC. It is not Medsafe approved and will have to be prescribed and dispensed in line with section 29 of the Medicines Act.
- Chemical: Dipyridamole
- Presentation: Cap modified-release 200 mg
- Brand Name: Dipyridamole – Strides
- Pharmacode: 2710919
- Subsidy: $55.13
- Quantity/per: 60
About Strides Dipyridamole
Important differences between the brands
| Feature | Pytazen SR | Strides |
|---|---|---|
| Presentation | Tablet (yellow) | Capsule (red and orange) |
| Strength | 150 mg | 200 mg |
| Release | Sustained release | Prolonged release |
While Strides is not approved by Medsafe. It is approved in the United Kingdom.
Datasheet for Strides dipyridamole | Medicines UK(external link)(external link)
Patient information for Strides dipyridamole | Medicines UK [PDF](external link)
- Dipyridamole discontinuation flyer Aug 25 [PDF 310 KB]
Prescribing and supplying an unapproved medicine
Section 29A of the Medicines Act 1981 allows for medicines that are not Medsafe approved to be prescribed and supplied to people.
We know supplying a medicine under section 29A is not ideal. In this case, however, this will allow patients to be able to access an appropriate treatment.
We apologise for any inconvenience this causes.
Prescriber and pharmacist requirements for section 29 medicines – Medsafe website (external link)
What patients need to know about unapproved medicines – Healthify website(external link)
What Pharmac has done
We have previously sought tenders for the ongoing supply of this product. Unfortunately, we have not had any bids for the on-going supply of dipyridamole. We have also engaged directly with various suppliers to see if dipyridamole could be made available for New Zealand. Unfortunately, these efforts have been unsuccessful, and dipyridamole will no longer be available in New Zealand once remaining stock has been used or expires.
We have removed all-at-once dispensing from the Pytazen SR brand to help preserve the remaining stock.
We have received clinical advice that people receiving dipyridamole may be able to be transitioned to other funded treatments.
We have recently funded ticagrelor to offer another alternative to people with minor stroke or high-risk TIA.
Decision to fund ticagrelor for people with minor stroke or high-risk TIA
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, Pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)
Pharmac is a government agency. We do not sell medicines or related products.


