Oxycodone immediate release tablets: Amneal is the new brand
Mundipharma, the supplier of the OxyNorm brand of oxycodone immediate release capsules, has left the New Zealand market. We have sourced an alternative brand, Oxycodone Amneal immediate release tablets.
11 October 2024 update
Delisting dates added for the OxyNorm capsules
Oxycodone Amneal immediate release tablets available now
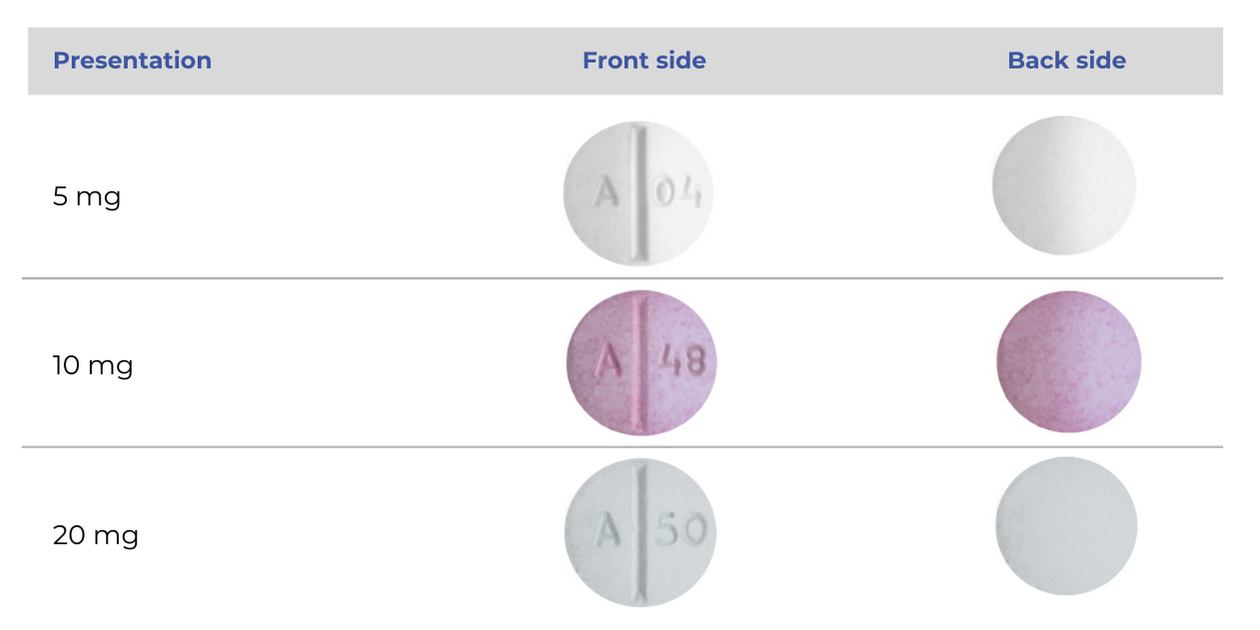
We have sourced supply of oxycodone immediate release tablets, called Oxycodone Amneal.
What’s changed?
- This brand is a tablet not a capsule.
- Oxycodone Amneal tablets have the same active ingredient as OxyNorm oxycodone capsules and they work in the body in the same way.
Oxycodone Amneal was listed on the Pharmaceutical Schedule from 1 July 2024, initially as a Section 29 medicine. It now has Medsafe approval.
| Strength | OxyNorm capsule Pharmacode |
Oxycodone Amneal tablet Pharmacode |
|---|---|---|
| 5 mg | 2179741 | 2681919 |
| 10 mg | 2179768 | 2681927 |
| 20 mg | 2179776 | 2681935 |
Schedule listings for Oxycodone Amneal immediate-release tablets(external link)
For people who take oxycodone immediate release tablets
Oxycodone is a strong opioid pain reliever. The Healthify website has great advice on what you need to know about taking opioids.
Opioid medicines overview – Healthify(external link)
If you take other forms of oxycodone (such as oxycodone controlled-release tablets) you might now be taking a tablet for both immediate and controlled release oxycodone.
- Amneal Oxycodone brand change A5 Flier (Aug24) [PDF 580 KB]
Review prescribing practice
We have received clinical advice that it may be appropriate for clinicians to review their prescribing practice around opioids.
Revisiting opioid use in New Zealand: how does your prescribing compare? BPACnz(external link)
Timeline
May 2024: supplier out of stock of OxyNorm 10 mg capsules
1 July 2024: Oxycodone Amneal 5 mg, 10 mg and 20 mg tablets listed on Pharmaceutical Schedule
July 2024: supplier out of stock of OxyNorm 5 mg capsules
October 2024: estimated out of stock of OxyNorm 20 mg capsules
1 October 2024: OxyNorm 10 mg capsule delisted
1 December 2024: OxyNorm 5 mg capsule delisted
1 March 2025: OxyNorm 20 mg capsule to be delisted
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


