Metoprolol: Your brand is changing
Between 1 November 2023 and 1 April 2024, your brand of metoprolol will be changing from Betaloc CR to Myloc CR.
What's changing?
Your brand of metoprolol is changing from Betaloc CR to Myloc CR. Your tablet will be a different shape and the packaging will change.
Medicines can look different, It's what's inside that's important [PDF, 403 KB]
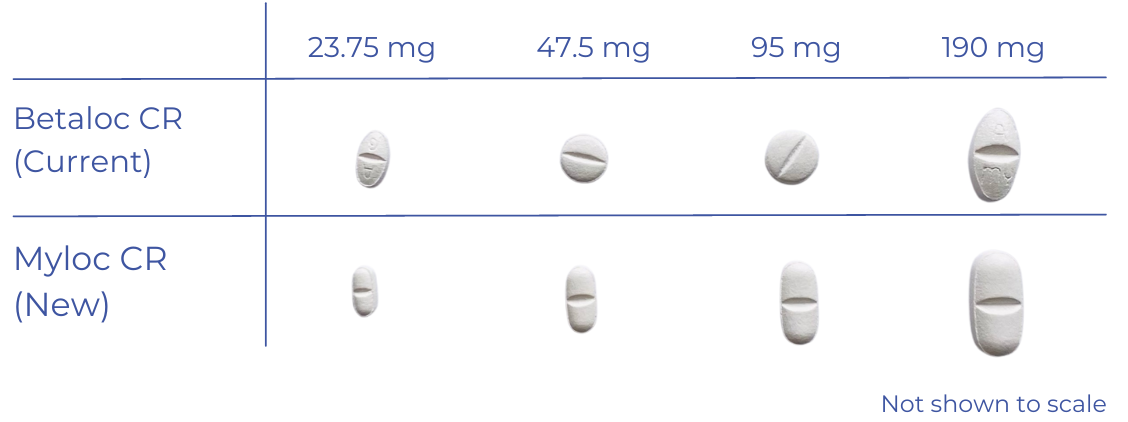
Brand change leaflet
- A5 Metoprolol Brand Change flier - November 2023 [PDF 86 KB]
- A4 Metoprolol Brand Change flier - November 2023 [PDF 126 KB]
Myloc CR works the same way
Myloc CR contains the same active ingredient in the same quantities as the Betaloc CR brand. It will work in your body the same way.
Medsafe has assessed the Myloc CR brand for safety, effectiveness, and quality. It meets the same standards as the Betaloc CR brand. It is approved for use in New Zealand.
Myloc CR Datasheet [PDF] - Medsafe(external link)
Myloc CR Customer Information Sheet [PDF] - Medsafe(external link)
Why is this happening?
Pharmac runs an annual tender on many of the medicines we fund. This tender gives suppliers an opportunity to offer already funded products for better, more competitive prices. The savings we make through the tender process frees up money so we can fund new treatments for people.
Following a recent tender, we made the decision to change from AstraZeneca's Betaloc CR brand to Viatris' Myloc CR brand.
This brand change is helping us fund more medicines for better health outcomes for all New Zealanders.
Who to contact
If you have any questions about this change, talk to your pharmacist or the person who prescribes your metoprolol. They know you and your clinical history, they can offer you the best advice.
If you have funding questions about this change or other treatments, email enquiry@pharmac.govt.nz


