COVID-19 antivirals: Access Criteria
Access criteria current as at 1 February 2024
On this page
Information for people with COVID-19
If you have, or suspect you have, COVID-19 and are at high risk of developing severe illness from COVID-19:
- test early
- contact your health care provider.
They are best placed to let you know what your treatment options are. Treatments must be started within short timeframes from onset of symptoms.
How to get COVID-19 antivirals – info.health.govt.nz(external link)
Check with your pharmacy, some pharmacies can dispense funded Paxlovid without a prescription. You will still need a clinical assessment. These medicines may not be right for everyone, even if you meet Pharmac’s eligibility criteria.
Access criteria for nirmatrelvir with ritonavir and remdesivir
Access criteria – from any relevant practitioner.
Approvals are valid for people where the prescriber confirms the person meets the following criteria and has endorsed the prescription accordingly:
All of the following:
- Person has confirmed (or probable) symptomatic COVID-19, or has symptoms consistent with COVID-19 and is a household contact of a positive case;
AND - Person’s symptoms started within the last 5 days (if considering nirmatrelvir with ritonavir) or within the last 7 days (if considering remdesivir);
AND - Person does not require supplemental oxygen as a result of their COVID-19 infection#;
AND - ANY of the following:
- Person is aged 65 years or over; or
- Person is Māori or Pacific ethnicity AND aged 50 years or over; or
- Person is aged 50 years or over AND has not completed a primary course^ of COVID-19 vaccination; or
- Person is immunocompromised* and not expected to reliably mount an adequate immune response to COVID-19 vaccination or SARS-CoV-2 infection, regardless of vaccination status; or
- Person has had a previous admission to Critical Care or High Dependency care directly as a result of COVID-19; or
- Person has Down syndrome; or
- Person has sickle cell disease; or
- Person receives Disability Support Services funded by Whaikaha - Ministry of Disabled People (previously Ministry of Health); or
- Person has pre-existing high risk due to a health condition and needs direct family, whānau or external disability care most days; or
- Person has pre-existing severe frailty and/or vulnerability due to one or more severe health conditions**; or
- Person has any combination of three or more high-risk factors for severe illness from COVID-19***;
AND
- Not to be used with other COVID-19 antiviral treatments.
Notes:
* Identifying people who are severely immunocompromised as per list hosted on the Pharmac website
** Health conditions that include severe or very advanced disease including, but not limited to, severe neurological, cardiovascular, renal and respiratory conditions
*** High risk factors for severe illness from COVID-19 as per list of hosted on Pharmac website
^ ‘primary course’ defined as receiving at least two courses of vaccination against COVID-19
# supplemental oxygen to maintain oxygen saturation >93%, or at or above baseline, for people with chronic resting hypoxia due to COVID-19 infection (excluding people with chronic resting hypoxia as a result of conditions other than COVID-19).
How to interpret the access criteria
We have developed an online tool to help you assess whether your patient is eligible for funded COVID-19 antiviral treatments.
Access criteria assessment tool
We have updated the heat maps to help healthcare professionals interpret criterion 4.11 of the access criteria and identify eligible patients.
We will continue to monitor the evidence as it becomes available, as well as available supply and update the criteria as required.
Heat maps to identify eligible patients under criteria 4.11 for for nirmatrelvir with ritonavir (Paxlovid) and remdesivir (Veklury)
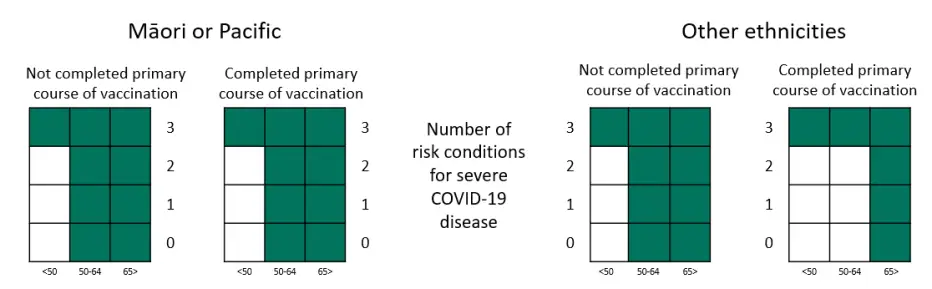
Table representation of the heat maps, showing numbers of high-risk medical conditions needed in criteria 4.11
| Age less than 50 |
Age between 50 and 64 |
Age over 65 |
||
|---|---|---|---|---|
| Māori or Pacific | Completed primary course | 3 | 0 | 0 |
| Not Completed primary course | 3 | 0 | 0 | |
| Other ethnicities | Completed primary course | 3 | 3 | 0 |
| Not Completed primary course | 3 | 0 | 0 |
The 3 required high-risk conditions could include multiple conditions which affect the same organ system or conditions affecting different organ systems.
People who meet one of criteria 4.4 to 4.10, do not need to have any added high risk medical conditions. Below are some example scenarios for eligibility:
Examples are based on the high risk medical conditions listed on our website as of 1 October 2023. These risk factors may change over time. Please refer this page for the most up-to-date high risk medical conditions.
Example 1: Person is Māori, aged 52 years old and has completed a primary course of vaccination.
This person meets criterion 4.2. If they meet the other criteria, then they are eligible for treatment.
Example 2: Person is of European ethnicity aged 64 years old, has completed a primary course of vaccination and has diabetes, a chronic lung condition, and a BMI over 35.
This person has three risk conditions (as on the Pharmac website) and therefore meets criterion 4.11. If they meet the other criteria, then they are eligible for treatment.
Example 3: Person is aged 35 years old, has completed a primary course of vaccination and has a very advanced neurological condition which has resulted in severe frailty.
This person meets criterion 4.10 If they meet the other criteria, then they are eligible for treatment.
Example 4: Person is aged 40 years old, has cerebral palsy and requires care from whānau five days a week.
This person meets criterion 4.9 If they meet the other criteria, then they are eligible for treatment.
Accessing supply of antiviral treatments (for pharmacies and hospitals)
Access to antiviral treatments will continue under the current arrangements that are in place.
The COVID-19 antiviral treatments are not accessed by a standard Special Authority. Instead, prescriptions must be endorsed by the prescriber confirming that the patient meets the Access Criteria. The Access Criteria will continue to be available on Pharmac’s website and linked to via Health Pathways. This approach allows us to easily make changes to the criteria if required in a timely manner.
Antiviral treatments are supplied to pharmacies and Health New Zealand | Te Whatu Ora hospitals at no cost, as they have been purchased directly by Pharmac | Te Pātaka Whaioranga.
Since 1 July 2024, COVID-19 treatments have been funded from New Zealand’s annual budget for medicines (the Combined Pharmaceutical Budget).
Nirmatrelvir with ritonavir (Paxlovid)
Paxlovid is listed in both in the Community Pharmaceutical Schedule and the Hospital Medicines List. Supply is available to order by both community pharmacies and Health NZ hospitals. This process is managed by Health NZ.
Community pharmacy
Supply to community pharmacies is currently managed through one wholesaler, ProPharma.
Paxlovid is listed with the XPharm restriction in the community. This is because Pharmac has purchased stock of this medicine directly. For funded dispensings, community pharmacies need to claim through Health NZ's Paxlovid Community Pharmacy Services programme.
Pharmacies should not charge people the standard $5 prescription co-payment for funded dispensings of Paxlovid.
Health NZ hospitals
Health NZ hospitals can order stock from OneLink as required.
Pharmacies no longer wishing to provide nirmatrelvir with ritonavir (Paxlovid)
Please consider:
- dispensing your remaining Pharmac purchased (Xpharm) Paxlovid stock before withdrawing from Paxlovid Community Pharmacy Services.
- alternately, return the Xpharm Paxlovid stock to Propharma as you would any other medicine.
Health care providers can find participating community pharmacies on the Community HealthPathways COVID-19 case management for adults(external link) webpage.
Note: You need a login to access
More information
COVID-19 treatment portfolio (includes distribution and stock availability)(external link)
Schedule listing for nirmatrelvir with ritonavir (Paxlovid)(external link)


