Decision regarding PHARMAC’s Implementation of Trans-Pacific Partnership (TPP) provisions and other Amendments to Application Processes
PHARMAC is pleased to announce that changes will be made to its Operating Policies and Procedures (OPP) and other changes concerning application processes.
This was the subject of a consultation document released on 5 September 2016. In summary, the effect of the decision is that:
- Section 2.1 of the OPP will be amended with immediate effect; and
the following changes would come into effect when TPP comes into force for New Zealand
- Sections 4.1 and 4.5 of the OPP will be amended
- a new decision-type ‘decline as proposed’ will be able to be applied to decisions; and
- an online product application tool and Product Application Assessment Record.
Details of the decision
Operating Policies and Procedures Section 2.1 Amendments to the Pharmaceutical Schedule
Pharmaceutical suppliers, clinicians, consumers, DHBs and any other interested parties may approach PHARMAC to suggest possible amendments to the Schedule, using the process described in the relevant funding application Guidelines. PHARMAC may amend the Schedule as it considers appropriate, including initiating amendments of its own accord. Possible amendments to the Schedule include (but are not limited to):
a. listing new pharmaceuticals
b. changing the terms on which a pharmaceutical is listed including;
a. changing guidelines or restrictions on prescribing and dispensing
b. changing the subsidy levels of pharmaceuticals as a result of PHARMAC adopting one of the strategies set out in section 3 or by any other means;
c. delisting pharmaceuticals or delisting part or all of a therapeutic group or sub-group;
d. changing packaging sizes and brand names;
e. changing the indications, formulations, presentations or any other feature of a listed pharmaceutical;
c. amending the basis on which pharmaceuticals are classified into therapeutic groups and sub-groups; or
d. publishing of information or requirements relating to the implementation of contracts for supply to DHB hospitals
Section 2.1 of the OPP takes effect immediately. The numbering and heading of the Operating Policies and Procedures section 2.1 will be amended as required in future editions of the Operating Policies and Procedures.
Operating Policies and Procedures Section 4.1 General
4.1.1 All applicants are encouraged to contact PHARMAC prior to making an application for funding for a chemical or biological entity to discuss that application.
4.1.2 The procedure to be followed in respect of an application for an amendment to the Schedule may vary depending on a number of factors, including (but not limited to):
a. the nature of the amendment (e.g., new listing, delisting, classification);
b. who has initiated the amendment (e.g., PHARMAC, supplier, interested parties) and whether it is the first time they have made this application;
c. the type of pharmaceutical being listed (e.g., a new medicine or a generic medicine, a medical device, related product, or related thing);
d. whether the amendment would result from an RFP, tender, listing contract or some other arrangement; ore. whether the amendment is a result of PHARMAC adopting a new strategy; or
f. any current funding arrangements in place for the same or competitor product.
4.1.3 PHARMAC may require a party initiating an amendment to the Schedule to provide in their application relevant information, including (but not limited to):
a. pharmacological information (forms, strength, indications, dosages, contra-indications etc);
b. therapeutic information (main therapeutic claims, advantages/ disadvantages when compared with other pharmaceuticals etc);
c. price information (proposed price, price overseas, other pricing proposals);
d. epidemiological information (number of people with the particular condition, number likely to be prescribed the pharmaceutical etc);
e. market information (expected sales etc);
f. detailed information on the costs and benefits of the pharmaceutical (e.g., reductions in expenditure; improvements in longevity and/or quality of life etc); and
g. information regarding packaging and pack sizes.
PHARMAC will decide what information it requires on a case by case basis. For example, less information may be required where a party proposes that PHARMAC list a generic pharmaceutical, as opposed to the listing of a new pharmaceutical.
4.1.4 Subject to PHARMAC’s right to prioritise its consideration of proposed amendments, PHARMAC is not bound to consider any proposed amendment until the party initiating the amendment has complied with all the conditions set by PHARMAC, including (but not limited to):
a. providing non-biased information;
b. setting out the basis for any estimates or assumptions made;
c. providing a synopsis on all material issues; and
d. providing comprehensive and detailed cost/benefit information.
4.1.5 All applications for amendments to the Pharmaceutical Schedule must be made in accordance with the Guidelines for Funding Applications to PHARMAC. For the avoidance of doubt, the Guidelines do not apply to responses to tenders, RFPs or other commercial proposals issued by PHARMAC.
4.1.6 PHARMAC will operate a TPP track for applications that meet the eligibility criteria in 4.1.7 and an Open track for all other applications for amendments to the Pharmaceutical Schedule.
4.1.7 An application for reimbursement and listing on the Pharmaceutical Schedule will only be eligible for the TPP track if it is:
4.1.7.1 an application from a pharmaceutical supplier (meaning an entity with the necessary rights in connection with the medicine for PHARMAC to be willing to enter into a contract for supply with that entity);
4.1.7.2 “formal and duly formulated” in terms of the Guidelines for Funding Applications to PHARMAC;
4.1.7.3 for a medicine as defined in the Medicines Act as at 4 February 2016 (not a medical device, related product, or related thing);
4.1.7.4 the first application to PHARMAC by that supplier (or a Predecessor Company) for that chemical or biological entity, and is NOT an application for an additional indication, or for a formulation, presentation, combination, or any other use of a chemical or biological entity that has been the subject of a previous application by that supplier (or its Predecessor Company) for reimbursement or listing on the Pharmaceutical Schedule;
4.1.7.5 for a medicine registered with Medsafe for all indications cited within the funding application; and
4.1.7.6 for an application seeking reimbursement for use of the medicine in the community (including oral cancer medicines).
4.1.8 The following applies to applications on the TPP track:
4.1.8.1 The supplier may make amendments to its application within 20 working days of initial submission. After that time, no further changes may be made to the application until the assessment stage has been completed. PHARMAC may choose whether to accept any changes proposed by the supplier after the assessment stage has been completed, taking into consideration whether there is likely to be sufficient time to make a Final Determination within the time specified under 4.1.8.2 as a consequence of the processing required.
4.1.8.2 A final decision will be made by PHARMAC within 30 months of an application meeting the requirements set out in 4.1.7 above being submitted unless an extension (or extensions) to the specific period of time is notified by PHARMAC to the supplier with reasons for the delay stated.
4.1.8.3 A supplier may apply for a review of a decision not to list a medicine where the application has been made under the TPP track (“TPP Review”). The TPP Review must be requested within 20 working days following notification of a decision not to list. The TPP Review is to be conducted by PHARMAC in accordance with the process determined by PHARMAC for this purpose.
4.1.8.4 No additional application will be accepted from a supplier for the same or a related indication for a medicine undergoing review until the TPP Review is completed. For the avoidance of doubt, a supplier can make an Open track application for an unrelated indication while a TPP Review is underway.
4.1.8.5 A supplier which has an application on the TPP track can withdraw its application at any time. Any subsequent application from that supplier in relation to the same chemical or biological entity would be dealt with as an Open track application.
4.1.9 Supplier applications not eligible for the TPP track are processed under the Open track, as are all applications from all other applicants.
4.1.9.1 Once an application is submitted under the Open track, PHARMAC may request further information. PHARMAC may decide not to consider an application until all requested information has been provided.
4.1.10 PHARMAC will make available information on the progress of applications in a timely and transparent manner.
Section 4.1 takes effect from the date the Trans-Pacific Partnership enters into force for New Zealand. The numbering and headings would be amended as required in future editions of the Operating Policies and Procedures;
Operating Policies and Procedures Section 4.5 Procedure for Listing a Pharmaceutical on the Pharmaceutical Schedule
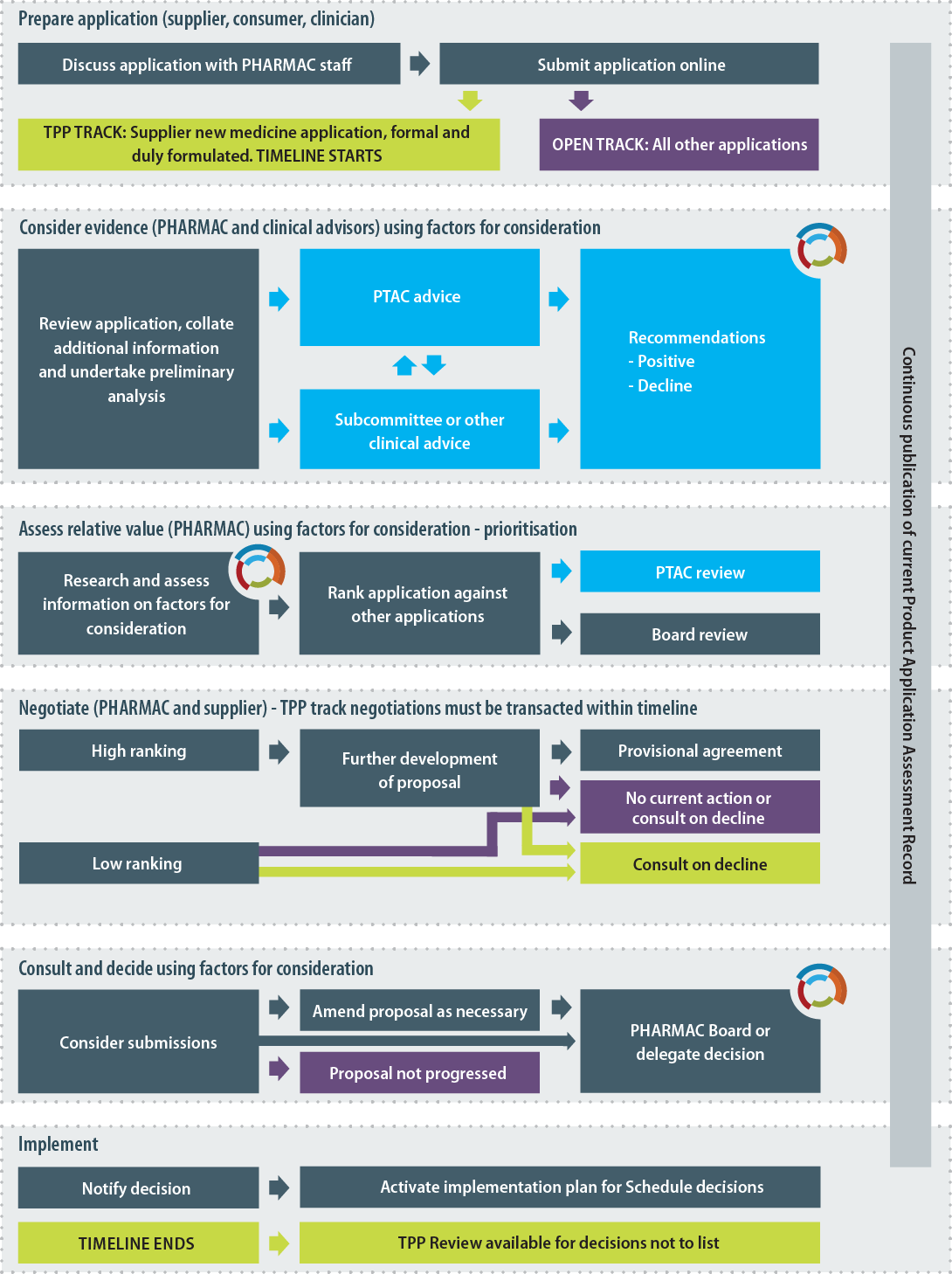
Note: This diagram provides a simplified, indicative guide to the process that PHARMAC will usually follow when listing a pharmaceutical on the Schedule. PHARMAC is not bound to follow the process set out in the diagram and may vary this process or adopt a different process where appropriate. PHARMAC provides a range of opportunities for applicants and other interested parties to engage with it regarding an application which is under assessment.
Section 4.5 takes effect from the date the Trans-Pacific Partnership enters into force for New Zealand. The numbering and headings would be amended as required in future editions of the Operating Policies and Procedures.
Other decisions that would come into effect when TPP comes into force for New Zealand
A new decision-type ‘decline as proposed’ will be able to be applied to decisions.
An online product application tool and Product Application Assessment Record.
Feedback received
We appreciate all of the feedback that we received and acknowledge the time people took to respond. All consultation responses received by 3 November 2016 were considered in their entirety in making a decision on the proposed changes. Most responses were supportive of the proposal, and the following issues were raised in relation to specific aspects of the proposal:
|
Theme |
Comment |
|
Some responders questioned why hospital medicines and medical devices might be excluded from the TPP track and the impact of changes to OPP section 2.1 on the TPP track. |
The content of 2.1 relates to all applications, regardless of track. Deletion of the content relating to hospital pharmaceuticals represents deletion of historic arrangements that predate the establishment of the Hospital Medicines List and the current structure of section H which includes hospital medical devices. As the changes to section 2.1 reflect a general tidy up of the OPP, the amended OPP will come into effect alongside other general OPP revisions, prior to any TPP-related changes. |
|
Some respondents questioned the definition of ‘pharmaceutical’ in the OPP. |
The definition of ‘pharmaceutical’ is the same as the NZPHD Act. |
|
While most responders were satisfied with the TPP track and Open track, suppliers and their industry representative group do not support the two tracks. However, in the event it did proceed, they did recommend some amendments to the OPP section 4.1. Most of these concerned greater clarity, a reduction in the specified period of time to complete applications, an extension to the time for the submission period of grace, the period of time within which a TPP Review must be requested, and an ability to make Open track applications for the same chemical or biological entity for unrelated indications where a TPP track application is under TPP Review. |
Various clauses on the OPP have been amended to aid clarity. The specified period of time for a TPP track application to be determined has been reduced from 36 months to 30 months. The submission period of grace for the TPP track has been extended from 10 to 20 working days. The period of time within which a TPP Review must be requested is 20 working days following final determination. The restriction on making new applications while undergoing a TPP Review has been limited to the same or related indications. These changes would come into effect when the TPP comes into force for New Zealand.
|
|
Most submitters were supportive of the other proposed changes such as the online application tool, the Product Application Assessment record and the ‘decline as proposed’ decision-type. Suppliers were keen to remain involved in the development of any information technology solutions. |
PHARMAC will proceed to implement a ‘decline as proposed’ decision-type across any track. PHARMAC will continue working with supplier representatives and other users to develop the online application tool and Product Application Assessment Record. These changes would come into effect when the TPP comes into force for New Zealand. |
|
Many responders expressed support for PHARMAC’s work and concern about possible adverse effects of TPP, possibly in terms of inequitable outcomes or treatment of applications. There was some concern that TPP would allow large overpriced suppliers to overrule PHARMAC’s agreements. |
PHARMAC intends to process all applications in the same way. The fixed period of time for a decision relates to TPP obligations, which affect certain supplier applications.
|
|
Some responders questioned whether the consultation was premature given TPP has not been ratified by partners. |
PHARMAC is acting under Ministerial Direction to have regard to the need to comply with New Zealand’s obligations in Annex 26-A of the TPP. The changes would only take effect when TPP comes into effect for New Zealand. |
|
Some responders questioned whether PHARMAC’s activities would still be subject to TPP general provisions including the potential for Investor State Dispute Settlement (ISDS) provisions. There was particular reference to Article 18.50 and 18.51 and implementation of their provisions requiring legislation that would conflict with the Patents Act 2013. It was claimed that ISDS provisions would apply to pharmaceutical investment.
|
New Zealand’s obligations under Annex 26-A of the TPP are not subject to TPP’s dispute settlement mechanisms, including ISDS. Like other New Zealand free trade agreements, however, some general provisions of TPP apply where PHARMAC is acting as part of the New Zealand Government. In respect to ISDS, where an entity or individual is deemed to be an investor under TPP rules, various safeguards have been included in TPP that protect the delivery of public services, including healthcare and PHARMAC’s role in funding pharmaceuticals. No changes are required to New Zealand law to implement articles 18.50 and 18.51 of the TPP. |
|
Some responders expressed concern about the financial impact on PHARMAC of additional TPP activity. There were concerns about the potential for increased legal action against PHARMAC or PHARMAC being forced to list a product that it didn’t think should be funded. There were suggestions that a fee should be charged to suppliers. |
Government’s National Interest Analysis estimated the cost for PHARMAC to implement TPP provisions. This means that resourcing would be provided to support implementation, including resources for the Legal Risk Fund. TPP does not preclude the imposition of charging for certain activities. |
More information
If you have any questions about this decision, you can email us at enquiry@pharmac.govt.nz or call our toll free number (9 am to 5 pm, Monday to Friday) on 0800 66 00 50.


