How to work with us
Procurement
Procurement processes are released by our procurement team on the New Zealand Government Electronic Tenders Service (GETS) via a Future Procurement Opportunity (FPO).
You can sign up to receive notifications for processes you are interested in. Pharmac’s processes are searchable under "Pharmaceutical Management Agency".
Contract Management
Every medicine and medical device supplier with an active agreement is assigned a Contract Manager. If you supply both medicines and devices you may have a Contract Manager for each category.
Contract Managers are generally contactable via phone or email, however in some instances written notification is required (such as when you’re expecting a supply issue).
There is also opportunity for suppliers to meet with Contract Managers online or over a phone call at more regular intervals. This encourages open communication between Pharmac and suppliers and builds better working relationships. This can be organised by either the Pharmac Contract Manager or the supplier and can be a good
opportunity to discuss items including ongoing supply issues or future goals and market projections.
When to get in contact with us
Given that clinicians and patients are relying on access to these medicines and medical devices, if there is anything we need to advise the market about, it’s important that we’re notified as soon as possible and kept in the loop.
In instances where there is a potential or upcoming supply issue, suppliers are obligated to notify us as soon as possible. This is so we have enough time to help you to ensure there is as little disruption to patients as possible – often we can provide advice and assistance on how to manage the issue and maintain continuity of supply.
- Changes to product specifications (including packaging, pack sizes, specifications)
- Pricing change requests
- Discontinuations
- Potential Supply Issues
Changes to medicines
Pharmac considers the clinical suitability of treatments prior to entering into supply agreements for pharmaceuticals. Before any changes are made to funded products, it is important that we are aware and supportive of these changes.
When making changes to medicines listed on the Pharmaceutical Schedule, a Notification of Product Change (NOPC) form should be completed. This includes both contracted and non-contracted products. For changes that have not been previously agreed with Pharmac, suppliers should discuss the change with their Pharmac Contract Manager before submitting this form.
Changes to medical devices
Pharmac has a separate process for managing device changes which includes listing of new brands, error correction, price changes and de-listing. If you are a medical device supplier and wish to progress a change to your product, please contact your Contract Manager who can provide advice and resources to facilitate this.
Getting devices onto the Hospital Medical Devices List
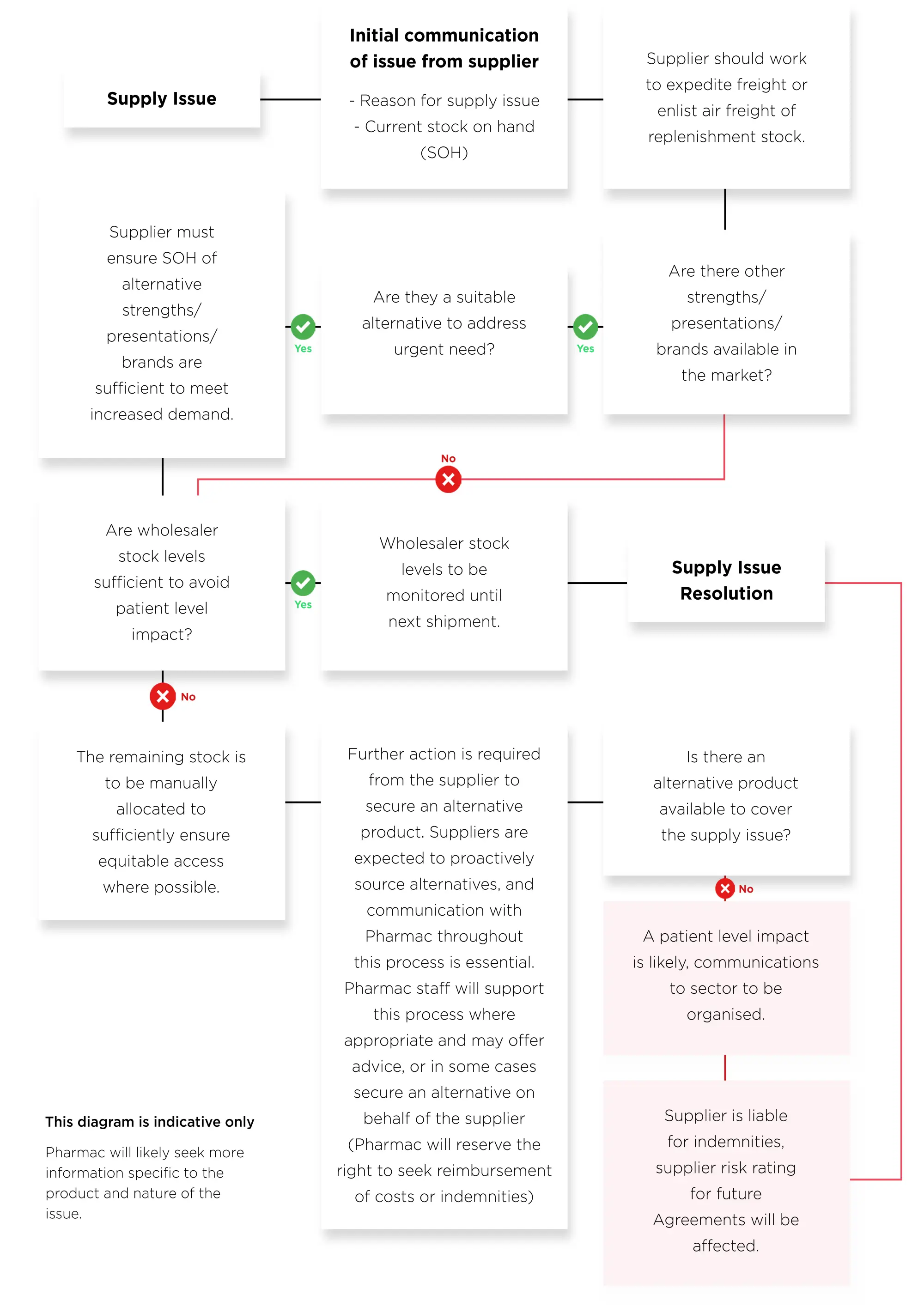
What information is important when there is a supply issue
We know that not everything can be planned for, and supply issues can arise with little notice. When dealing with supply issues, it is important that we all act in a timely manner. It is important to give us as much notice as possible so that we can consider suitable alternative treatments and notify relevant people early on.
After you have noticed a potential supply issue, it is important to understand the likely impact on the market. This information will also assist us in deciding if we need to make any changes to the Schedule to limit the impact on New Zealanders.
The diagram on the following page is a summary of the information that is necessary for you to provide to us as soon as you become aware of a potential supply issue. Some of this information may need to be sought from other parties.