Prochlorperazine (Buccastem): Supply issue
Due to a manufacturing issue, prochlorperazine tab 3 mg buccal (Buccastem 340537) is out of stock.
15 March 2024 update
Stock of Max Health's temporary section 29 brand, Brown and Burk, is available to order. Pharmac aims to have this product listed in the Pharmaceutical Schedule from 22 March 2024.
Supply of the currently listed section 29 product, Max Health (2658801) has run out at the supplier level but there may be stock in the supply chain.
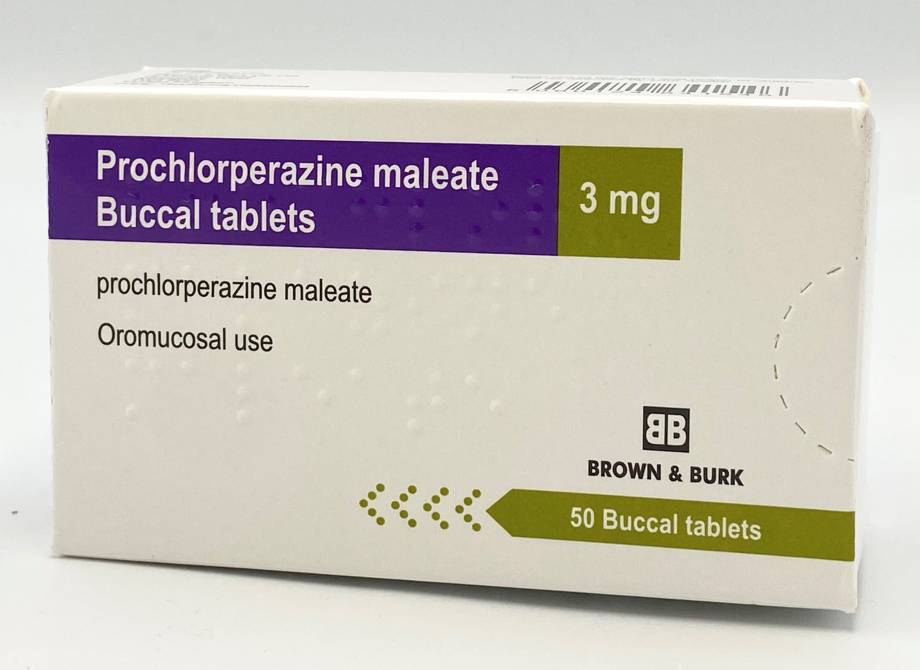
(20/3 picture of box added)
About the issue
Max Health are out of stock of Buccastem (pharmacode 340537). The supply issue was initially caused by a certification issue at the manufacturing plant. Resupply has been delayed because another supplier had discontinued a product needed for Buccastem.
Alternative products
Brown and Burk temporary replacement
Stock of the Brown and Burk prochlorperazine buccal tablets has arrived with Max Health. Pharmac is aiming to list it, subject to approval, from 22 March 2024.
It is available to order from the supplier.
Max Health temporary brand replacement
The Max Health alternative was listed on 1 July 2023, as at 7 March 2024 supplies had run out at the supplier, but there will still be stock in the supply chain. This alternative is chemically identical to Buccastem and will be supplied under Section 29 with wastage rules applied. It was listed in the Pharmaceutical Schedule from 1 July 2023 as follows:
- Chemical: Prochlorperazine
- Presentation: Tab 3 mg buccal
- Brand: Max Health
- Price: $5.97 ($30.00)
- Pack size: 50
- Pharmacode: 2658801
Schedule listing for prochlorperzine(external link)
Resupply
We are seeking confirmation from Max Health on a resupply date for the Buccastem brand of buccal prochlorperazine.
Who to contact
If you have any questions about the Buccastem supply issue, contact Max Health: 09 815 2664
You can email us on enquiry@pharmac.govt.nz
(Please include details on which product you are emailing about).
Sign up to our email list for regular emails about supply issues and more(external link)


