Mythbusting Pharmac
Where we dispel some misunderstandings about what Pharmac does and how we do it.
Myth: Pharmac cares more about money than lives.
Truth: The people who work at Pharmac want all New Zealanders to live longer, healthier, happier lives. We care about money only because we have a finite budget which we need to manage carefully. We want to get the best out of this money for the best health outcomes for New Zealanders.
In 2024/25, our medicines budget got a large boost from the Government to $1.5 billion. We funded or widened access to 66 medicines, vaccines, and related products for New Zealanders.
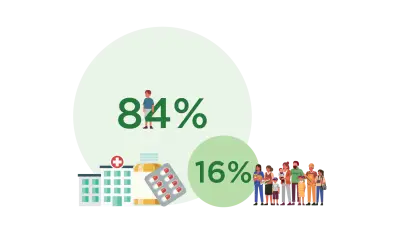
Myth: Pharmac only funds cheap bulk medicines
Truth: Nearly 4 million people receive funded medicines each year. About 10% of those people account for 84% of the spending on medicines.
These are expensive medicines to treat conditions, such as autoimmune conditions and cancer.
Over time, the percentage of funds used by the top 10% of people is increasing. 6 years ago 10% of people accounted for 79% of spending.
Myth: These medicines are available in 80 other countries, but not in New Zealand
Truth: Medicines might be ‘available’ in other countries, but it doesn’t mean they are Government funded.
In New Zealand, if a medicine is funded, generally, 100% of its cost is covered by the government.
On average in the OECD, governments and compulsory insurance only covered 58% of community medicine costs. Most of the rest is coming out of patients’ pockets.
In Germany and France, government and compulsory health insurance cover 82% or more of medicine costs. By contrast, some countries had a much higher out-of-pocket expense: Chile (70%), Poland (65%), and Latvia (55%).
Pharmaceutical expenditure: 2025 OECD Indicators(external link)
New Zealand has some of the lowest out-of-pocket health expenses in the OECD.
Myth: Australians have access to lots more funded medicines
Truth: Australia does fund more medicines. However, unlike New Zealand, they do not have a fixed budget.
In the 2022/23 financial year the Australian Pharmaceutical Budget spend was AU$17 billion. In 2022/23, Pharmac's budget was $1.186 billion.
Expenditure and Prescriptions Report 1 July 2022 to 30 June 2023 - PBS Australia(external link)
Pharmac is legally required to stay within the budget the Government has allocated for medicines, vaccines and related products
In addition, Australians pay more for each prescription, generally about AU$30. Most New Zealanders only pay $5.
While no system is perfect, the New Zealand model means cost is less of a barrier for people to access the medicines they need.
About the Australian Pharmaceutical Benefit System(external link)
Myth: Because Pharmac gives suppliers 95% of the New Zealand market, we are more likely to have stock issues.
Truth: New Zealand has fewer stock issues than Australia in general. When we give suppliers sole access to the funded market, we also make them responsible for ensuring that medicine is available. There can be financial penalties for suppliers who fail to keep medicines in stock.
The contracts we have with suppliers has meant we have had fewer issues reach consumers. Generally, we are able to find replacements for medicines with a supply issue.
Medicine notices of brand changes and supply issues on our website
Myth: Pharmac buys and sells medicines.
Truth: Pharmac doesn’t buy medicines. We just decide which ones the government will pay for. Pharmacies buy medicines from wholesalers, who work with suppliers and pharmaceutical companies. Health New Zealand | Te Whatu Ora then reimburses pharmacies at the rate we agreed with the pharmaceutical company.
Vaccines (except the influenza vaccine) and COVID-19 treatments are managed differently.
Myth: Pharmac refuses to fund new drugs
Truth: We are committed to funding medicines that are proven to make a difference for people. Price is not the only issue with many new medicines. Our clinical advisers tell us whether any new medicine has the evidence to back up the claims made by pharmaceutical companies.
In 2022/23 we funded 20 new medicines and widened access to 22 other medicines.
An estimated 364,954 more people benefited from our 2022/23 funding decisions.
Our 2024 funding boost has meant we've been able to progress many funding decisions.
Myth: Pharmac only funds the cheapest medicines.
Truth: When negotiating with pharmaceutical companies, we consider a range of factors. Price is one of these. However, we also look at things like taste, packaging, and a medicine’s overall impact on the health sector. For example, how do people take it? Is it a pill or do they need to see a nurse or doctor for an injection?
We have the Tender Clinical Advisory Committee (TCAC). It is made up of GPs, pharmacists, nurses, and other experts. As part of the tender process, they examine every brand of medicine that might be funded. They check the taste, the feel, how easy it is to get into the package.
The annual tender is one way that we get better prices for medicines.
Myth: Pharmac funds ‘knock off’ medicines that aren’t as good as ‘brand name’ versions.
Truth: Funded medicines must be approved by Medsafe. If we fund a ‘generic’ brand, it is as safe and effective as the original medicine.
Some medicines are very complex, called biologicals. There can even be differences between batches made by the same company. So when we fund the same medicine made by a different company, there can be some small differences – but no more difference than between batches. These medicines are called 'biosimilars'.
Always talk to a health professional if you notice side effects from any medicine.


