Consultation on proposed 2021/22 Invitation to Tender: For suppliers
Te Pātaka Whaioranga, Pharmac, is seeking feedback from pharmaceutical suppliers and interested parties on the proposed 2021/22 Invitation to Tender (ITT)
Pharmac is seeking feedback from pharmaceutical suppliers and interested parties on:
- A proposal to tender certain pharmaceuticals for principal supply;
- The implications of awarding Principal Supply Status; and
- Commercial proposals as an alternative to tendering.
Pharmac welcomes all feedback on the draft 2021/22 Tender. Feedback received by the deadline may be considered by the Tender Medical Evaluation Subcommittee of PTAC and would be considered by the Pharmac Board (or its Delegate, where applicable) prior to making a decision on this proposal.
Download a PDF version of this consultation
- Consultation document - suppliers pdf [PDF 184 KB]
Feedback should be submitted by the following dates; late feedback may not be considered:
Alternative commercial proposal responses due by:
4 pm (New Zealand Time), Wednesday 11 August 2021
All other consultation responses due by:
4 pm (New Zealand Time), Monday 16 August 2021
Feedback should be provided by submitting an email to the Tender Analysts:
Email: tender@pharmac.govt.nz
Feedback we receive is subject to the Official Information Act 1982 (OIA) and we will consider any request to have information withheld in accordance with our obligations under the OIA. Anyone providing feedback, whether on their own account or on behalf of an organisation, and whether in a personal or professional capacity, should be aware that the content of their feedback and their identity may need to be disclosed in response to an OIA request.
We are not able to treat any part of your feedback as confidential unless you specifically request that we do, and then only to the extent permissible under the OIA and other relevant laws and requirements. If you would like us to withhold any commercially sensitive, confidential proprietary, or personal information included in your submission, please clearly state this in your submission and identify the relevant sections of your submission that you would like it withheld. PHARMAC will give due consideration to any such request.
Details of the proposed 2021/22 Tender
In general, the proposed 2021/22 Tender process would be similar to the 2020/21 Tender in that the 2021/22 Tender would result in awarding Principal Supply Status (PSS) (previously Sole Supply Status and/or Hospital Supply Status).
Packaging preferences
We have historically indicated a preference for the pack size of Tender Items supplied in the community to be either a 30-day or 90-day pack, as this improves dispensing efficiency and reduces wastage. In the coming years we are intending to put greater emphasis on the appropriateness of both packaging type and pack size, with a preference for bottle packs for tablets and capsules where possible. In this Tender we have identified specific packaging preferences for individual products in Schedule 2, and we expect to expand this to a much wider range of products in future Tenders.
We are seeking feedback on the composition of the draft 2021/22 Tender. This is still under development and may change before it is taken to the Board (or its Delegate) for approval and subsequently issued. At this stage, but depending on the extent of any changes, Pharmac does not intend to send out further drafts for consultation.
In addition to Alternative Commercial Proposals (discussed below), we seek comments on all sections of the draft 2021/22 Tender, in particular on:
- An indication of any pharmaceuticals, whether or not they are included in Schedule Two of the draft 2021/22 Tender, that you consider should be tendered, and the reasons for that view. If you wish, you may provide a non-binding confidential indication of the price or price range that you might be able to offer for a line item or group of line items you wish to have tendered;
- An indication of any pharmaceuticals, whether or not they are included in Schedule Two of the draft 2021/22 Tender, that you consider would be inappropriate to tender, and the reasons for that view, including any contractual constraints or patent protection that could restrict Pharmac from awarding a tender on a particular pharmaceutical;
- Your views on whether any product included in Schedule Two might have more than 5% of patients needing to access an alternative brand;
- Feedback on any unresolved Tender Bid(s) from previous tenders that you consider should remain open for acceptance. Please note that some currently unresolved Tender Bids may be resolved prior to the consultation deadline and the final 2021/22 Tender being issued.
Draft Invitation to Tender
A complete copy of the draft 2021/22 Tender, including the proposed terms and conditions which successful tender bids would be subject to, is available on our website.
Download the 2021/22 draft Invitation to Tender [PDF, 601 KB]
The draft 2021/22 Tender comprises the following sections:
Schedule 1: Definitions and interpretation
Schedule 2: The list of pharmaceuticals proposed for tender* #
Download Schedule 2: Products to be tendered [XLSX, 32 KB]
Download Supplement to Schedule 2 (includes pharmacodes) [XLSX, 26 KB]
Schedule 3: The tender process (for both hospital and community tender bids)
Schedule 4: Contract terms for Principal Supply Status for both community and hospital supply
Schedule 5: Additional contract terms for Principal Supply Status for community supply
Schedule 6: Additional contract terms for Principal Supply Status for hospital supply
Schedule 7: Additional special terms for particular pharmaceuticals
*The units provided in Schedule Two consist of market data for the year ended 30 June 2021. The figures included are indicative only and are provided on the basis set out in clause 1.3 of Schedule 2 of the draft 2021/22 Tender.
#The final list of products, which may change following consultation, would be released as part of the 2021/22 Tender, following Board (or its Delegate) approval. You may provide feedback on the inclusion of any additional pharmaceuticals after the 2021/22 Tender has been issued, and any such feedback would be considered by the Board (or its Delegate) before making a final decision on any product, provided that any feedback is given prior to the tender close date in late 2021.
Key proposed inclusions:
|
Summary of change |
Schedule and clause references in the draft 2021/22 ITT |
|---|---|
|
Further clarification with additional/amended definitions |
Schedule 1 Brand Allowance Pharmaceutical In-Use Shelf-Life Offer Letter Shelf-Life Tender Submission Form Total Brand Allowance Pharmaceutical Volume |
|
Ability to extend supply arrangements either to the community market or hospital market where a Tender Bid was received for the alternative market. |
Schedule 3 clauses 1.4 and 1.5 |
|
Clarification that only Tender Bids that have been entered and submitted via the electronic form are considering conforming Tender Bids (i.e. Tender Bids submitted bypassing the data entry format would not be considered Conforming). |
Schedule 3 clause 6 (ii) |
|
Schedule 4 clause 1.5 |
|
|
Additions to the arrangements after the End Date. |
Schedule 5 clause 1.5 (iii), and Schedule 6 clause 1.3 (iv) |
|
Warranty the price is inclusive of all costs relating to the Tender Item. |
Schedule 5 clause 3.3 |
|
Clarification to price and volume data access. |
Schedule 6 clause 10 (c) |
Additional Special Terms
Intra-uterine copper devices
Additional Special Terms have been included in the draft 2021/22 Tender contract for intra-uterine copper devices (Schedule Seven). This clause requires any potential suppliers to offer education, training and support resources to patients and healthcare professionals. This clause also outlines the additional information suppliers must provide relating to their Tender Bid. The full Additional Special Terms for intra-uterine copper devices can be found in the draft 2021/22 Tender contract on the Pharmac website.
Long-acting filgrastim
Long-acting filgrastim has been included in the draft 2021/22 Tender. Pharmac would consider bids for any long-acting filgrastim product (e.g. pegfilgrastim, pegfilgrastim biosimilars, and lipegfilgrastim) for this market; all of which would be subject to the same access criteria. Pharmac reserves the right to award the tender to one long-acting filgrastim product for the entire market.
Please note: in response to feedback from the Ministry of Health Haematology Working Group (HWG) during the early stages of the COVID-19 pandemic in 2020, Pharmac amended the access criteria for pegfilgrastim. Access was widened to patients when used for the prevention of neutropenia in patients undergoing high risk chemotherapy for cancer (febrile neutropenia risk greater than or equal to 5% [was previously 20%]). This change was intended to be a temporary health sector resource saving measure with the intention that access criteria would revert to the previous threshold of 20% on 1 December 2020.
A decision on whether to revert to the 20% threshold was deferred in August 2020(external link). A decision on whether to maintain or amend the access criteria is expected to be made over the coming months subject to clinical advice (please note that this may not be as restrictive as the original criteria).
The figures and table below are provided to give an indication of the potential market size under both access criteria scenarios while a decision is still pending. Please note the information is approximate and indicative only. Pharmac makes no representation as to the accuracy of this information or as to the level of sales or likely sales of long-acting filgrastim and, while Pharmac has taken all reasonable care in preparing the information set out below, it accepts no liability for any errors or omissions in the information. Pharmac is not obliged to notify you in the event of any change to the figures below:
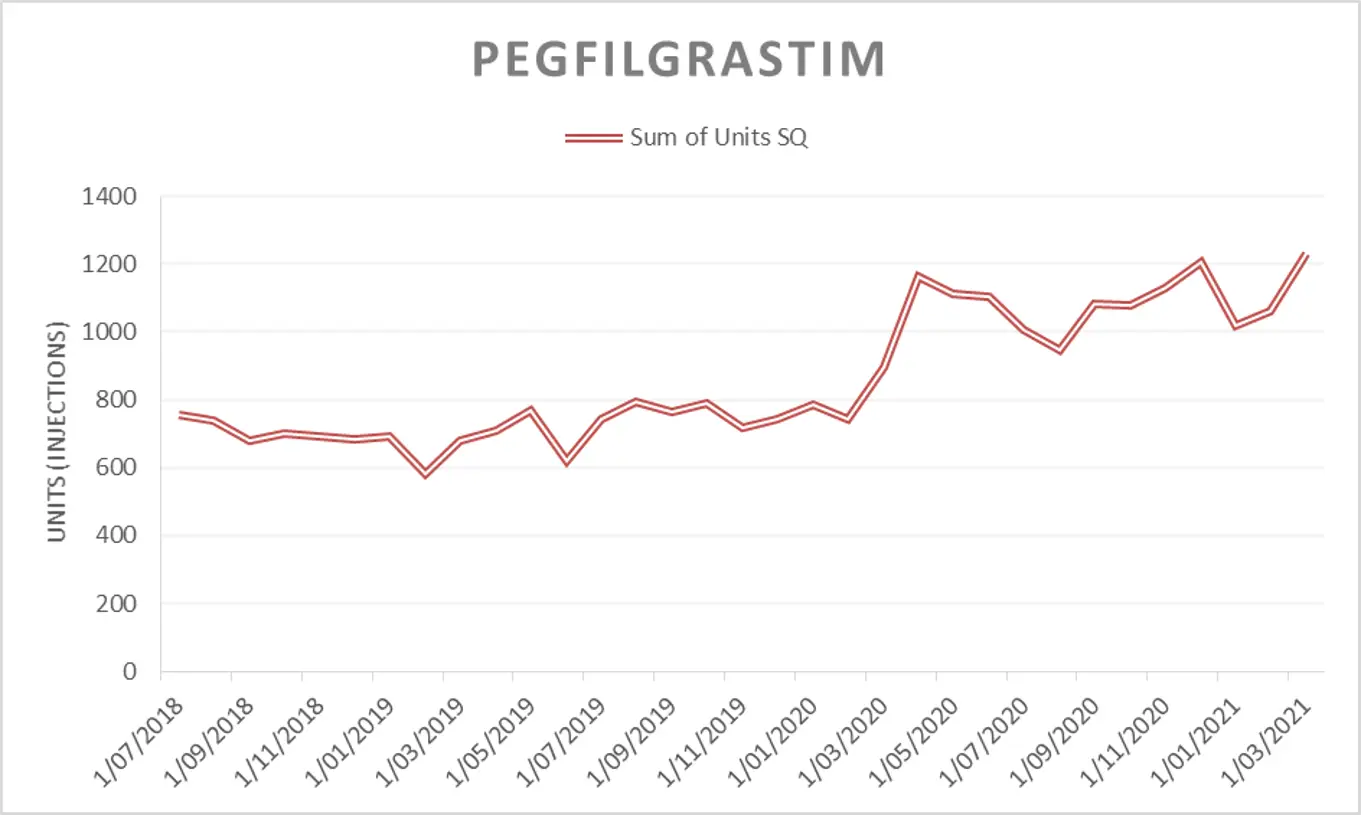
|
Usage of pegfilgrastim by financial year* |
||
|
|
Units |
Gross Cost |
|
2018/19 |
8,303 |
$8,967,240 |
|
2019/20 |
10,363 |
$11,192,040 |
|
2020/21 |
11,917 |
$12,870,360 |
|
* Financial years run from 1 July to 30 June. |
||
Key dates and timeframes for the 2021/22 Tender
The timelines for the 2021/22 Tender are envisaged to be similar to the 2020/21 Tender; we propose to release the final 2021/22 Invitation to Tender in early November 2021 and consequently the closing date for tender submissions would be mid December 2021. The proposed timeline is outlined in the following table:
|
Date |
Event |
|---|---|
|
19 July 2021 |
Consultation with suppliers, medical groups and interested parties on the proposed pharmaceutical list and draft 2021/22 Tender. |
|
11 August 2021 |
Final date for receipt of Alternative Commercial Proposals (ACPs) to tendering by Pharmac. |
|
16 August 2021 |
Final date for all consultation to be received. |
|
August/September 2021 |
Pharmac considers feedback from consultation, negotiates with suppliers over any ACP proposals it considers would meet Pharmac’s Factors for Consideration, and enters into provisional contracts with suppliers where appropriate. |
|
September 2021 |
Meeting of the Tender Medical Evaluation Subcommittee of PTAC to consider clinical issues in relation to the proposed Tender list. |
|
September/October 2021 |
Consultation and decisions on Alternative Commercial Proposals. |
|
Early November 2021 |
Issuing of the 2021/22 Tender. |
|
16 December 2021 |
Invitation to Tender closes. |
|
From end of January 2022 |
Announcements on 2021/22 Tender decisions will commence. |
Unresolved Tender Bids
We intend to review any unresolved Tender Bids from the 2018/19 ITT, 2019/20 ITT and 2020/21 Tenders prior to issuing the 2021/22 Tender. The following Tender Bids remain unresolved:
2018/19 Invitation to Tender
|
Chemical Name |
Line Item |
|---|---|
|
Teriparatide |
Inj 250 mcg per ml |
2019/20 Invitation to Tender
|
Chemical Name |
Line Item |
|---|---|
|
Acetazolamide |
Tab 250 mg |
|
Amoxicillin clavulanate |
Grans for oral liq amoxicillin 125 mg with potassium clavulanate 31.25 mg per ml |
|
Amoxicillin clavulanate |
Grans for oral liq amoxicillin 250 mg with potassium clavulanate 62.5 mg per 5 ml |
|
Bupivacaine hydrochloride |
Inj 2.5 mg per ml, 20 ml ampoule |
|
Carbimazole |
Tab 5 mg |
|
Carmellose sodium |
Eye drops 1% |
|
Clobazam |
Liq |
|
Docetaxel |
Inj 20 mg |
|
Docetaxel |
Inj 80 mg |
|
Ephedrine |
Inj 3 mg per ml, 10 ml prefilled syringe |
|
Erlotinib hydrochloride |
Tab 100 mg |
|
Erlotinib hydrochloride |
Tab 150 mg |
|
Ethinyloestradiol with levonorgestrel |
Tab 30 mcg with levonorgestrel 150 mcg |
|
Ethinyloestradiol with levonorgestrel |
Tab 20 mcg with levonorgestrel 100 mcg |
|
Exemestane |
Tab 25 mg |
|
Glyceryl trinitrate |
Inj 5 mg per ml, 10 ml ampoule |
|
Ivabradine (current access) |
Tab 5 mg |
|
Ivabradine (current access) |
Tab 7.5 mg |
|
Ivabradine (widened access) |
Tab 5 mg |
|
Ivabradine (widened access) |
Tab 7.5 mg |
|
Lamivudine |
Tab 300 mg |
|
Levosimendan |
Inj 2.5 mg per ml, 5 ml |
|
Metaraminol tartrate |
Inj 0.5 mg per ml, 10 ml |
|
Metaraminol tartrate |
Inj 0.5 mg per ml, 5 ml prefilled syringe |
|
Metaraminol tartrate |
Inj 0.5 mg per ml, 10 ml prefilled syringe |
|
Morphine |
Inj 20 mg per ml |
|
Morphine |
Inj 50 mg per 5 ml |
|
Morphine |
Inj 100 mg per 5 ml |
|
Morphine |
Inj 10 mg per ml, 1 ml |
|
Morphine |
Inj 15 mg per ml, 1 ml |
|
Morphine |
Inj 30 mg per ml, 1 ml ampoule |
|
Mupirocin |
Intra-nasal ointment 2% |
|
Noradrenaline |
Inj 0.06 mg per ml, 50 ml vial |
|
Noradrenaline |
Inj 0.12 mg per ml, 50 ml vial |
|
Noradrenaline |
Inj 0.1 mg per ml, 50 ml syringe |
|
Ondansetron hydrochloride |
Inj 2 mg per ml, 2 ml |
|
Ondansetron hydrochloride |
Inj 2 mg per ml, 4 ml |
|
Piperacillin with tazobactam |
Inj 4 g with tazobactam 500 mg |
|
Rosuvastatin |
Tab 5 mg |
|
Rosuvastatin |
Tab 10 mg |
|
Rosuvastatin |
Tab 20 mg |
|
Rosuvastatin |
Tab 40 mg |
|
Talc |
Dusting Powder BP |
|
Thiamine hydrochloride |
Tab 50 mg |
2020/21 Invitation to Tender
|
Chemical Name |
Line Item |
|---|---|
|
Atracurium besylate |
Inj 10 mg per ml, 2.5 ml |
|
Atracurium besylate |
Inj 10 mg per ml, 5 ml |
|
Baclofen |
Tab 10 mg |
|
Brimonidine tartrate with timolol maleate |
Eye drops 0.2% with timolol maleate 0.5% |
|
Carboplatin |
Inj 10 mg per ml, 45 ml |
|
Cefalexin monohydrate |
Grans for oral liq 25 mg per ml |
|
Cefalexin monohydrate |
Grans for oral liq 50 mg per ml |
|
Chlorhexidine gluconate |
Mouthwash 0.2% |
|
Clonidine |
Tab 25 mcg |
|
Colchicine |
Tab 500 mcg |
|
Dapsone |
Tab 100 mg |
|
Dapsone |
Tab 25 mg |
|
Daptomycin |
Inj 350 – 500 mg |
|
Docusate sodium with sennosides |
Tab 50 mg with sennosides 8 mg |
|
Enoxaparin sodium |
Inj 20 mg per 0.2 ml |
|
Enoxaparin sodium |
Inj 40 mg per 0.4 ml |
|
Enoxaparin sodium |
Inj 60 mg per 0.6 ml |
|
Enoxaparin sodium |
Inj 80 mg per 0.8 ml |
|
Enoxaparin sodium |
Inj 100 mg per ml, 1 ml |
|
Enoxaparin sodium |
Inj 120 mg per 0.8 ml |
|
Enoxaparin sodium |
Inj 150 mg per ml, 1 ml |
|
Eplerenone (current access) |
Tab 25 mg |
|
Eplerenone (current access) |
Tab 50 mg |
|
Eplerenone (widened access) |
Tab 25 mg |
|
Eplerenone (widened access) |
Tab 50 mg |
|
Ethambutol hydrochloride |
Tab 400 mg |
|
Felodipine |
Tab long-acting 2.5 mg |
|
Fenofibrate |
Cap/tab 48 mg |
|
Fenofibrate |
Cap/tab 145 mg |
|
Fentanyl |
Inj 10 mcg per ml, 10 ml syringe |
|
Fentanyl |
Inj 20 mcg per ml, 100 ml bag |
|
Fentanyl |
Inj 20 mcg per ml, 50 ml syringe |
|
Flumazenil |
Inj 0.1 mg per ml, 5 ml |
|
Fluorouracil sodium |
Inj 50 mg per ml, 20 ml |
|
Fluorouracil sodium |
Inj 50 mg per ml, 100 ml |
|
Fosfomycin (current access) |
Powder |
|
Fosfomycin (widened access) |
Powder |
|
Hydroxocobalamin |
Inj 1 mg per ml |
|
Hydroxychloroquine sulphate |
Tab 200 mg |
|
Lanreotide |
Inj 60 mg per 0.5 ml, 0.5 ml syringe |
|
Lanreotide |
Inj 90 mg per 0.5 ml, 0.5 ml syringe |
|
Lanreotide |
Inj 120 mg per 0.5 ml, 0.5 ml syringe |
|
Liquid paraffin with white soft paraffin |
Liquid paraffin 50% with white soft paraffin 50% ointment (pack size 100 g or less) |
|
Liquid paraffin with white soft paraffin |
Liquid paraffin 50% with white soft paraffin 50% ointment (pack size greater than 100 g) |
|
Lisinopril |
Tab 5 mg |
|
Lisinopril |
Tab 10 mg |
|
Lisinopril |
Tab 20 mg |
|
Methotrexate |
Inj 7.5 mg prefilled syringe |
|
Methotrexate |
Inj 10 mg prefilled syringe |
|
Methotrexate |
Inj 15 mg prefilled syringe |
|
Methotrexate |
Inj 20 mg prefilled syringe |
|
Methotrexate |
Inj 25 mg prefilled syringe |
|
Methotrexate |
Inj 30 mg prefilled syringe |
|
Midodrine |
Tab 2.5 mg |
|
Midodrine |
Tab 5 mg |
|
Naloxone hydrochloride |
Inj 400 mcg per ml, 1 ml |
|
Neostigmine metisulfate |
Inj 2.5 mg per ml, 1 ml |
|
Nitrofurantoin |
Tab 50 mg |
|
Nitrofurantoin |
Tab 100 mg |
|
Noradrenaline |
Inj 0.06 mg per ml, 50 ml syringe |
|
Noradrenaline |
Inj 0.1 mg per ml, 100 ml bag |
|
Noradrenaline |
Inj 0.12 mg per ml, 100 ml bag |
|
Noradrenaline |
Inj 0.16 mg per ml, 50 ml syringe |
|
Oil in water emulsion |
Crm (pack size 100 g or less) |
|
Oil in water emulsion |
Crm (pack size greater than 100 g) |
|
Oxaliplatin |
Inj 100 mg |
|
Oxycodone hydrochloride |
Inj 10 mg per ml, 1 ml |
|
Oxycodone hydrochloride |
Inj 10 mg per ml, 2 ml |
|
Oxycodone hydrochloride |
Inj 50 mg per ml |
|
Pancreatic enzyme |
Cap 150 mg |
|
Pancreatic enzyme |
Cap 300 mg |
|
Pemetrexed |
Powder for infusion, 100 mg |
|
Pemetrexed |
Powder for infusion, 500 mg |
|
Prednisolone |
Rectal Foam 10 – 20% |
|
Ramipril |
Cap/tab 1.25 mg |
|
Ramipril |
Cap/tab 2.5 mg |
|
Ramipril |
Cap/tab 5 mg |
|
Ramipril |
Cap/tab 10 mg |
|
Sugammadex |
Inj 100 mg per ml, 2 ml |
|
Sugammadex |
Inj 100 mg per ml, 5 ml |
|
Sunitinib (current access) |
Cap 12.5 mg |
|
Sunitinib (current access) |
Cap 25 mg |
|
Sunitinib (current access) |
Cap 37.5 mg |
|
Sunitinib (current access) |
Cap 50 mg |
|
Sunitinib (widened access) |
Cap 12.5 mg |
|
Sunitinib (widened access) |
Cap 25 mg |
|
Sunitinib (widened access) |
Cap 37.5 mg |
|
Sunitinib (widened access) |
Cap 50 mg |
|
Teicoplanin |
Inj 400 mg |
|
Terlipressin |
Inj 1 mg per 8.5 ml ampoule |
|
Terlipressin |
Inj 0.2 mg per ml, 5 ml |
|
Ticagrelor |
Tab 90 mg |
|
Vecuronium |
Inj 10 mg |
Should any unresolved Tender Bids be declined prior to the release of the 2021/22 Tender, Pharmac would consider re-tendering those pharmaceuticals when the 2021/22 Tender is issued. Unresolved Tender Bids have not been included in the draft pharmaceutical list (Schedule Two).
Products not currently listed the Pharmaceutical Schedule
The following products included in Schedule Two of the draft 2021/22 Tender are not currently listed in the Pharmaceutical Schedule:
|
Chemical Name |
Line Item |
|---|---|
|
Benzoyl peroxide |
Gel/crm/soln/lotion 2.5% - 5% |
|
Erwinia asparaginase |
Inj |
|
Levothyroxine |
Oral liq |
|
Mifepristone with misoprostol |
Mifepristone 200 mg tablet x 1 and misoprostrol 200 mcg tablet x 4 [combination pack] |
|
Paracetamol with codeine |
Tab paracetamol 500 mg with codeine 30 mg |
|
Prasugrel |
Tab 5 mg |
|
Prasugrel |
Tab 10 mg |
|
Telmisartan |
Tab/Cap 40 mg |
|
Telmisartan |
Tab/Cap 80 mg |
|
Telmisartan with hydrochlorothiazide |
Tab 40 mg with hydrochlorothiazide 12.5 mg |
|
Telmisartan with hydrochlorothiazide |
Tab 80 mg with hydrochlorothiazide 12.5 mg |
|
Telmisartan with hydrochlorothiazide |
Tab 80 mg with hydrochlorothiazide 25 mg |
|
Tolterodine |
Tab 1 mg |
|
Tolterodine |
Tab 2 mg |
Electronic Tender (eTender) system
The 2021/22 Tender will be distributed via Pharmac’s electronic tendering portal. The portal requires companies to register for a user account and details of how to register will be distributed prior to the release of the final 2021/22 Invitation to Tender. Please let us know if the contact details for the person responsible for submitting tender bids have changed for your company by sending an email to the Tender Analysts at tender@pharmac.govt.nz by 4 pm (New Zealand time), Friday 24 September 2021.
Alternative Commercial Proposals
Pharmac seeks any Alternative Commercial Proposals (ACPs) to tendering that you may wish to submit. An ACP may, for example, offer price reductions on one set of pharmaceuticals in return for Pharmac agreeing to defer tendering on another group of pharmaceuticals for a period.
Please note the following points apply to ACPs for both the community and DHB hospital markets:
- ACPs should include at least one item listed in Schedule Two of the draft 2021/22 Tender;
- ACPs may include more than one line item and may include pharmaceuticals not listed in Schedule Two of the draft 2021/22 Tender;
- ACPs should not include any items subject to an unresolved tender;
- ACPs may seek Pharmac’s agreement to defer tendering or application of reference pricing for a period of time for any pharmaceutical, whether or not it is listed in Schedule Two of the draft 2021/22 Tender;
- ACPs may not propose awarding Principal Supply Status in the community or DHB Hospitals, or any other form of sole or exclusive supply;
- Pharmac reserves the right:
- not to accept any ACPs; and/or
- not to provide reasons for the acceptance or non-acceptance of any ACP; and/or
- to enter into an agreement or arrangement that differs in a material respect from that envisaged in this letter.
ACPs are due by 4 pm (New Zealand Time), Wednesday 11 August 2021. Pharmac may not consider any ACPs that are submitted after this date.
Usage data for ‘PCT only’ injectable products
The table below contains ‘PCT only’ usage data for items included in the 2021/22 Tender. These volumes are approximate and indicative only. Pharmac makes no representation as to the accuracy of these figures or as to the level of sales or likely sales of any tender item.
|
Chemical |
Total usage (mg)* |
|
Bortezomib |
22,210 |
|
Fludarabine |
16,300 |
|
*Usage in mg, for period between 1 January 2020 to 31 December 2020 |
|


