Updated Access Criteria for antiviral COVID-19 treatments
Current access criteria
As our response to the COVID-19 pandemic evolves, we are updating access criteria to treatments.
What we’re doing
We're pleased to announce that following the arrival of molnupiravir in New Zealand we have made the decision to widen access to three antiviral medicines used to treat COVID-19 from 5 May 2022.
- nirmatrelvir with ritonavir (supplied under the brand name Paxlovid)
- molnupiravir (supplied under the brand name Lagevrio) and
- remdesivir (supplied under the brand name Veklury).
These changes mean that more New Zealanders will be able to access these treatments.
Nirmatrelvir with ritonavir and remdesivir are currently available in New Zealand and are being used in the treatment of early COVID-19. These access criteria changes will be implemented from 5 May 2022 to align with molnupiravir becoming available for people with COVID-19 in New Zealand.
Access Criteria have been developed with advice from our clinical expert advisors and other stakeholders. The updated criteria widen access to antiviral treatments to more people who are at increased risk of severe COVID-19, including people with Down Syndrome, Sickle cell disease, people over 65 years of age and people who have not completed a full course of vaccination against COVID-19.
We have also heard from healthcare professionals that managing multiple antiviral treatments for COVID-19 with different access criteria is difficult. To address this, we have made a decision to apply the same access criteria to the three antiviral treatments available in New Zealand for the treatment of COVID-19. We have also clarified the wording of the criteria and have developed resources to make them easier to understand.
People with COVID-19 who are immunocompromised or have comorbidities that put them at risk of severe COVID-19 will be eligible for treatment, as before.
No changes have been made to the distribution arrangements for these treatments, more information about New Zealand’s COVID-19 treatments portfolio, availability of the treatments and how to access them is available on our website.
This decision to widen access to antiviral treatments for COVID-19 follows previous decisions by Pharmac to widen access to remdesivir and to list nirmatrelvir with ritonavir and molnupiravir on the Pharmaceutical Schedule subject to access criteria from 1 April 2022.
Detail about this decision
The Access Criteria
The following Access Criteria will apply to nirmatrelvir with ritonavir (Paxlovid), molnupiravir (Lagevrio) and remdesivir (Veklury) from 5 May 2022. Prescriptions must be endorsed by the prescriber confirming that the patient meets the Access Criteria.
Access criteria – from any relevant practitioner.
Approvals are valid for patients where the prescribing clinician confirms the patient meets the following criteria and has endorsed the prescription accordingly:
All of the following:
- Patient has confirmed (or probable) symptomatic COVID-19, or has symptoms consistent with COVID-19 and is a household contact of a positive case;
AND - Patient’s symptoms started within the last 5 days (if considering nirmatrelvir with ritonavir or molnupiravir) or within the last 7 days (if considering remdesivir);
AND - Patient does not require supplemental oxygen#;
AND - Either:
- The patient meets ONE of the following:
- Patient is immunocompromised* and not expected to reliably mount an adequate immune response to COVID-19 vaccination or SARS-CoV-2 infection, regardless of vaccination status; or
- Patient has Down syndrome; or
- Patient has sickle cell disease; or
- Patient has at least FIVE of the following factors:
- Any combination of high-risk medical conditions for severe illness from COVID-19 identified by the Ministry of Health** (with each individual condition counting as one factor)
- Māori or any Pacific ethnicity (counts as one factor)
- Patient is aged 65 years and over (counts as two factors, or three if patient has not completed a full course of vaccination), or is 50 years and over (counts as one factor)
- Patient has not completed a full course of vaccination*** (counts as one factor);
AND
- The patient meets ONE of the following:
- Not to be used in conjunction with other COVID-19 antiviral treatments.
Notes:
Consider molnupiravir or remdesivir if nirmatrelvir with ritonavir is unsuitable or unavailable.
* As per Ministry of Health criteria of ‘severe immunocompromise’ for third primary dose
** People with high risk medical conditions identified by the Ministry of Health
*** ‘Fully Vaccinated’ defined as per the Ministry of Health definition. [link removed as document no longer available 16/8/22]
# Supplemental oxygen to maintain oxygen saturation >93% or at or above baseline for patients with chronic resting hypoxia
How to interpret the access criteria?
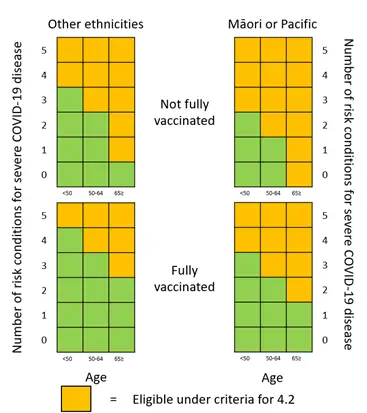
We have prepared heat maps to help healthcare professionals interpret criterion 4.2 of the access criteria and identify eligible patients. These heat maps along with other resources have been developed to assist with interpretation of the access criteria and will be available on our website alongside the access criteria from 5 May 2022.
Some factors in the criteria are now weighted and may be counted more than once. This reflects the increased risk of severe COVID-19 for people aged 65 years and over and those who have not completed a full course of vaccination against COVID-19.
We will continue to monitor the evidence as it becomes available, as well as available supply and update the criteria as required.
Table representation of the heat map to indicate the number of risk conditions required (as per criteria 4.2.1) to meet criteria 4.2 depending on other factors
| FACTORS | Age less than 50 |
Age between 50 and 64 |
Age 65 and over |
|
|---|---|---|---|---|
| Other ethnicities | Fully vaccinated | 5 | 4 | 3 |
| Not fully vaccinated | 4 | 3 | 1 | |
| Māori or any Pacific ethnicity | Fully vaccinated | 4 | 3 | 2 |
| Not fully vaccinated | 3 | 2 | 0 | |
For clarity, no specific factors (under criterion 4.2) are required to access these treatments. A person with any combination of at least five factors from 4.2.1. 4.2.2, 4.2.3 and 4.2.4 would meet criteria 4.2. This could include the factors named in the criteria (4.2.2, 4.2.3 or 4.2.4) or be any combination of the high-risk conditions listed on the Ministry of Health website (4.2.1).
Some examples of scenarios for eligibility:
Please note these examples have been drafted as per the risk factors listed on the Ministry of Health website as of 7 April 2022. These risk factors may change over time. Please refer to the Ministry of Health website for the most up to date risk factors.
Example 1: Patient has severe mental illness, diabetes that isn’t well controlled, has chronic lung disease, hypertension which isn’t well controlled and a BMI of 40.
This patient has five risk condition (as on the Ministry of Health website) and therefore meets criterion 4.2. If the patient meets the other criteria the patient is eligible for treatment.
Example 2: Patient is aged 55 and has not completed a full course of vaccination, is obese (BMI 36), has chronic lung disease and a serious heart condition.
This patient has five factors and therefore meets criterion 4.2. If the patient meets the other criteria the patient is eligible for treatment.
Example 3 Patient is aged 67 and fully vaccinated, has chronic kidney disease, hypertension and a BMI of 37. This patient has five factors and therefore meets criterion 4.2. If the patient meets the other criteria the patient is eligible for treatment.
Example 4 Patient is aged 73 and has not completed a full course of vaccination and has a chronic neuromuscular disease. This patient has five factors and therefore meets criterion 4.2. If the patient meets the other criteria the patient is eligible for treatment.
Example 5: Patient is immunocompromised and is not expected to reliably mount an adequate immune response to COVID-19 vaccination or SARS-CoV-2 infection, regardless of vaccination status. This patient meets criterion 3. If the patient meets the other criteria the patient is eligible for treatment.
Information for patients
If you have, or suspect you have COVID-19 and are at high risk of developing severe illness from COVID-19, test early and please get in touch with your health care provider. They are best placed to let you know what your treatment options are.
Accessing supply of antiviral treatments
Access to antiviral treatments will continue under the current arrangements that are in place.
The antiviral COVID-19 treatments are not accessed via a standard Special Authority. Instead, prescriptions must be endorsed by the prescriber confirming that the patient meets the Access Criteria. The Access Criteria will continue to be available on Pharmac’s website and linked to Health Pathways. This approach allows us to easily make changes to the criteria if required in a timely manner.
Antiviral treatments are supplied to pharmacies and DHB Hospitals at a cost of $0 as they have been purchased directly by Pharmac. COVID-19 treatments are funded from a dedicated budget allocated by the Government. That means COVID-19 treatment costs do not come from the annual budget for New Zealand’s medicines (the Combined Pharmaceutical Budget).
Nirmatrelvir with ritonavir and molnupiravir
Nirmatrelvir with ritonavir and molnupiravir will continue to be listed in Section B and Part II Section H of the Pharmaceutical Schedule.
Supply of nirmatrelvir with ritonavir and molnupiravir is available to order only by selected community pharmacies and DHB Hospitals. This process is being managed by the Ministry of Health.
Supply to community pharmacies is currently managed through one wholesaler, ProPharma and DHB Hospitals can order from Onelink as required. More deliveries will continue to arrive throughout 2022 and the access criteria have been designed to help to support prescribers to target treatment to those most in need and most likely to benefit. The XPharm rule applies to these treatments in the community as Pharmac has purchased these medicines directly. Community pharmacies are not able to claim subsidy through normal claiming systems as alternative funding arrangements have been established.
Nirmatrelvir with ritonavir and molnupiravir have been purchased directly by Pharmac and there will be no standard dispensing fee or patient co-payment. Pharmacies will be reimbursed by COVID-19 Care in the Community funding through DHBs. Information regarding the claiming mechanism for this payment to Pharmacies will be available from DHBs.
Remdesivir
Currently remdesivir is not listed on in the Pharmaceutical Schedule as it has been supplied to DHBs under special arrangements. We intend to list remdesivir in Part II Section H of the Pharmaceutical Schedule from 1 June 2022. Remdesivir would be listed subject to the same restriction as nirmatrelvir with ritonavir and molnupiravir.
Access to remdesivir will continue as it is now. Supply is managed through one wholesaler, OneLink. DHB hospitals can order stock direct from Onelink. Wholesale stock is held and distributed to DHB Hospitals by Onelink in Auckland.
Level 1 hospitals are able to order and administer remdesivir and DHB Hospitals are able to provide remdesivir to both inpatients and outpatients.
DHBs will not be invoiced for supply because this stock has been purchased directly by Pharmac.
For more information about distribution and stock availability please visit our main COVID-19 web page.
Where to get more information
For the most up to date information regarding all COVID-19 treatments – please check our website.
If you have any questions about this decision, you can email us at enquiry@pharmac.govt.nz; or call our toll free number (9 am to 5 pm, Monday to Friday) on 0800 660 050.


