Eltroxin (levothyroxine) 50 mcg and 100 mcg tablets: Medicine update
Aspen NZ, the supplier of Eltroxin (levothyroxine), has informed Pharmac that the 50 mcg and 100 mcg tablets are changing.
Update 22 August 2024
A brand switch fee will be available for pharmacists from 1 September 2024 until 30 November 2024 for the Eltroxin brand of levothyroxine. While this has not been a brand change, the brand switch fee is being applied to support pharmacists and recognise their time counselling patients on the Eltroxin product changes.
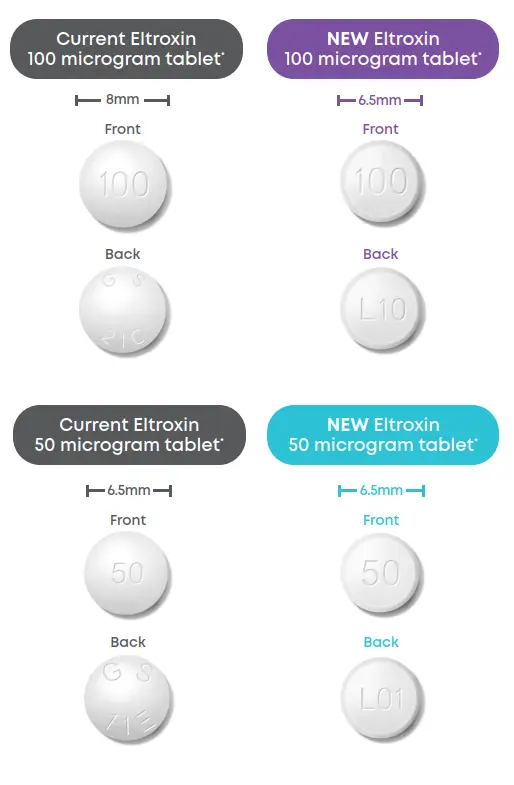
What’s happening?
Your medicine, Eltroxin 50 mcg and 100 mcg (levothyroxine), is changing. It will look different, but it will work the same way.
It is being made in the same place, with the same ingredients, as the old one.
How is the 100 mcg tablet changing?
The new Eltroxin 100 microgram tablet contains all the same ingredients as your current tablet. The manufacturing site is also the same.
There is the same amount of active ingredient (medicine) in each tablet.
There has been a reduction in the quantity of other ingredients that are used to make the medicine into a tablet (inactive ingredients), resulting in a new smaller tablet.
The tablet shape and tablet imprint has also changed, so it will look a little different.
How is the 50 mcg tablet changing?
The new Eltroxin 50 microgram tablet will also have a different tablet shape and tablet imprint.
However, both the active (medicine) and inactive ingredients of Eltroxin 50 microgram remain exactly the same.
The manufacturing site is also the same.
Why is this happening?
Aspen NZ is making these changes so your medicine will be the same as the medicine (Eltroxin) in Australia. Australia has been using these Eltroxin tablets since 2015.
When will this happen?
The Eltroxin tablet changes will happen as the current tablet supply runs out.
- Supply of the current Eltroxin 50 mcg tablets is expected to run out by mid-June.
- Supply of the current Eltroxin 100 mcg tablets is expected to run out by early August.
There will be a cross-over period from June to September 2024 where the current Eltroxin tablets available from pharmacies will run out and the new Eltroxin tablets will be available.
Medsafe has reviewed the new Eltroxin tablets
Medsafe has evaluated and approved the new version of medicine to ensure it meets international standards for quality, and that it works in the body in the same way.
If you have any questions or concerns about the changes to Eltroxin, you should talk with your doctor or pharmacist.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


