Brevinor 1/28 (Ethinyloestradiol with norethisterone): Supply issue resolved
There was a supply issue affecting Brevinor 1/28 oral contraceptive (Pharmacode 303542)
Update 10 January 2025
Resupply of the Brevinor 1/28 brand has arrived in New Zealand. It may take a few weeks for stock to move through the supply chain. The approved Alyacen 1/35 product remains available.
Affected product
There has been a supply issue with Brevinor 1/28 Day oral contraceptive due to an unexpected delay in sourcing the active ingredient, norethisterone:
- Chemical: Ethinyloestradiol with norethisterone
- Presentation: Tab 35 mcg with norethisterone 1 mg
- Brand: Brevinor 1/28
- Pharmacode: 303542
- Subsidy: $12.25
- Measure / Qty: per 84
Schedule listing for Brevinor 1/28(external link)
Three-month dispensing
All-at-once (stat) dispensing does not currently apply to this product. People can only collect three-months' supply at a time. We will reconsider all-at-once dispensing once the stock situation is resolved.
Alternative product
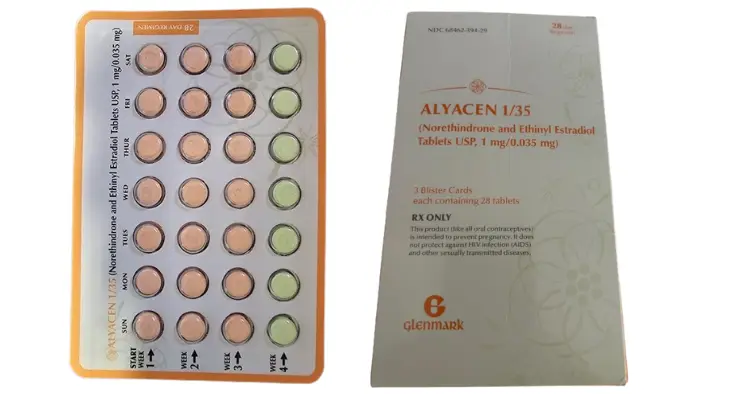
Alyacen 1/35 (pharmacode 2688441) will be funded from 1 October 2024 and a brand switch fee will apply. As of 18 October 2024, it is provisionally Medsafe-approved. The section 29 flag in the Schedule will be removed for 1 December 2024.
Alyacen 1/35 contains the same active ingredients in the same quantities as Brevinor 1/28. However, it uses the American name for norethisterone, which is norethindrone.
A5 flier about the temporary brand change
- Brevinor 1/28 | Supply issue brand change [PDF 297 KB]
Expected resolution
The supplier, Pfizer, expects supply to return to normal by early February 2025.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


