Bee venom (Venox): Packaging change
The supplier has changed the way bee venom allergy treatment initiation packs are packaged, so it now requires reconstitution.
What's changing?
The Initiation Kit for bee venom allergy treatment (pharmacode 2596822) has changed. Previously it came with five vials, each vial containing the active ingredient with the diluent. The new package has six vials, one vial with the active ingredient, one vial with solvent, and instructions on how to dilute it into the subsequent vials.
When will this happen
The new kit was listed from 1 February 2025 (Pharmacode: 2698773). The five-vial kit was delisted on 1 May 2025. It was short-dated.
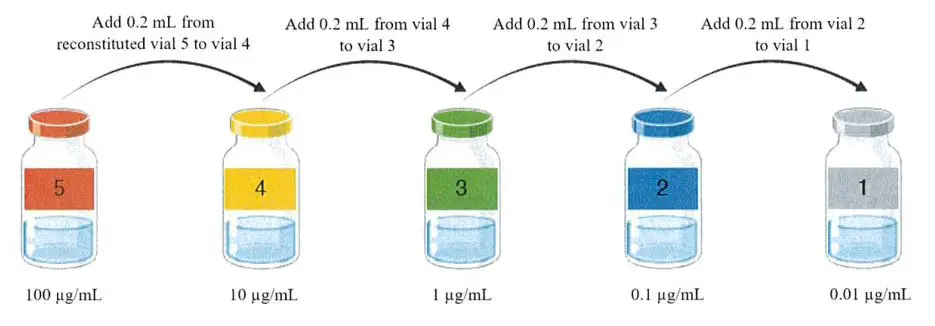
Reconstitution process
Follow the guidelines on how to reconstitute and administer Venox. Venox must be administered by trained health care professionals in places that are ready to manage any anaphylactic shock.
The manufacturer letter has instructions for making up the right doses of bee venom.
- Venox Jan 25 Manufacturer letter [PDF 275 KB]
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


