Proposal for funded multiple sclerosis treatments
We’re proposing some changes to the way that multiple sclerosis treatments are funded, as well as widening access to the funded treatments, from 1 March 2021.
What we’re proposing
In summary:
- applications to the Multiple Sclerosis Treatment Assessment Committee (MSTAC) would no longer be required and funded multiple sclerosis treatments would be accessed by a standard Special Authority application in the same way as many other funded medicines.
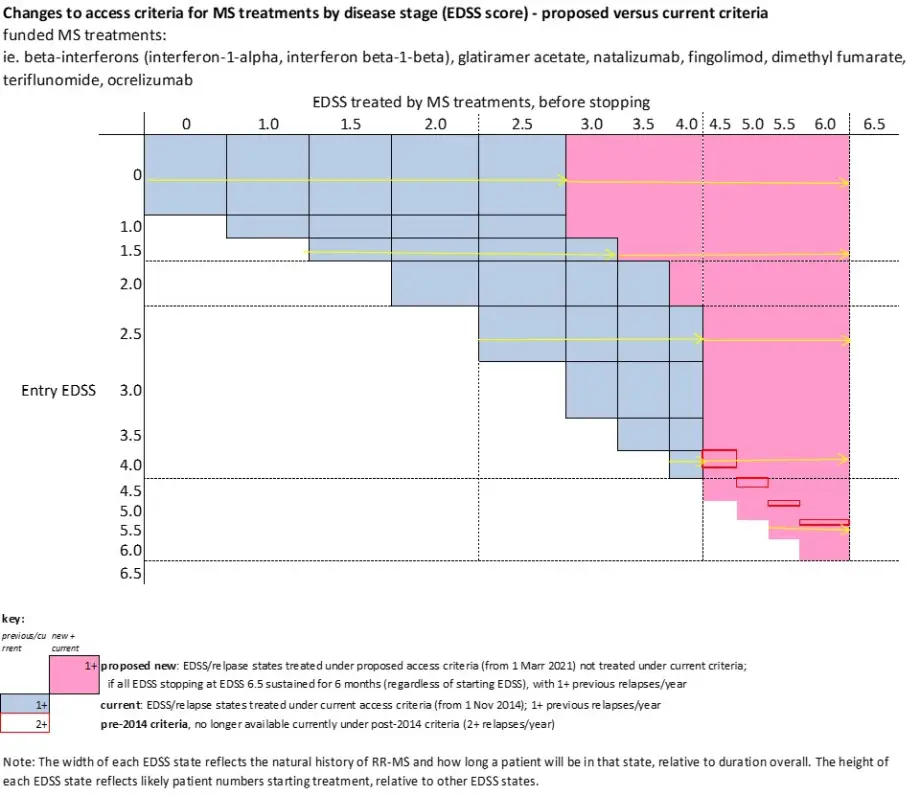
- access to funded multiple sclerosis treatments would be widened for people with an Expanded Disability Status Scale (EDSS) score of 0 to 6.0 (inclusive) meaning treatment would continue to be funded for patients while their EDSS is 6.0 or below.
The funded multiple sclerosis treatments are natalizumab (Tysabri), fingolimod (Gilenya), the beta interferons (interferon beta-1-beta (Betaferon), interferon beta-1-alpha (Avonex)), glatiramer acetate (Copaxone), teriflunomide (Aubagio), dimethyl fumarate (Tecfidera) and ocrelizumab (Ocrevus).
Consultation closes at 5 pm on Monday, 21 December 2020, and feedback can be emailed to mstaccoordinator@pharmac.govt.nz.
What would the effect be?
For patients
All patients using funded multiple sclerosis treatments would continue to stay on their funded treatment and most would be able to stay on their treatments for longer – up to, and including, an EDSS of 6.0. An EDSS of 6.0 means that someone can walk 100m with or without rest and/or assistance (including a cane, crutch or brace).
The proposed transition to standard Special Authority would also improve access for patients starting and continuing treatment, as it aims to reduce the burden placed on applicants and patients by enabling neurologists to complete assessments and gain approvals for funding faster and more easily.
The small number of patients with current approvals under older eligibility criteria that allow them to stay on treatment above EDSS of 6.0 would be ‘grand-parented’ and would be granted special approvals by PHARMAC to ensure that they would be able to remain on their current treatments with their current stopping criteria. This applies to 10 patients. We would communicate directly with the clinicians treating these 10 patients to inform them of the process to apply for an annual renewal.
For pharmacies and DHBs
There would be no significant impacts for pharmacies or DHBs from this proposal, other than the increased use of funded treatment due to patients staying on treatment for longer.
For prescribers
The proposal would not change which prescribers are eligible to apply for funded multiple sclerosis treatments; applications would continue to be made by neurologists and general physicians.
Currently, applications for funded multiple sclerosis treatments are managed by the MSTAC using specific entry and stopping criteria. If this proposal is approved, neurologists and general physicians would be able to apply for initial and renewal Special Authority approvals through the online Electronic Special Authority system. Applications would then be processed via the standard Special Authority process.
In almost all cases, patients with current MSTAC approvals would be automatically granted new standard Special Authority approvals, to ensure funded access to treatment continues for these patients. There are also 10 patients with approvals under older eligibility criteria that allow them to stay on treatment above EDSS of 6.0; these would be ‘grand-parented’ and would be granted special approvals by PHARMAC to ensure that they would be able to remain on their current treatments with their current stopping criteria. We would communicate directly with the clinicians of the 10 patients to inform them of the process to apply for an annual renewal. This transition process would ensure that all patients who are currently receiving funded treatment would continue to be eligible.
Neurologists and general physicians would continue to be able to delegate the writing of prescriptions for people with multiple sclerosis to the person’s GP.
Applying the standard Special Authority approval approach to multiple sclerosis treatments, combined with the proposed changes to the criteria, would allow funded access to these treatments to be managed in a more streamlined way. It would also improve access to these treatments as this transition aims to reduce the burden placed on clinicians by allowing annual assessments to be carried out more easily and there would be faster approval for funding.
The transition would also include widening of access to funded multiple sclerosis treatments to people with an EDSS score from 0 to 6.0 (inclusive), which for most patients would mean they could stay on a funded treatment for longer.
The Special Authority would apply to each and all of the currently funded MS treatments combined, as one single set of criteria (rather than as separate sets of criteria for each MS treatment, as occurs currently). This would allow clinicians to switch their patient’s treatment to a different chemical if clinically appropriate, without needing to apply for a new initial Special Authority approval.
Who we think will be interested
- People with multiple sclerosis, and their whānau, family, and caregivers
- Multiple Sclerosis New Zealand and others supporting individuals with multiple sclerosis
- Neurologists, neurology nurses, general physicians, general practitioners, and other health professionals involved in the management and treatment of multiple sclerosis
- Pharmacies and DHBs
- Pharmaceutical suppliers
Background
There are currently eight funded multiple sclerosis treatments: natalizumab (Tysabri), fingolimod (Gilenya), the beta interferons (interferon beta-1-beta (Betaferon), interferon beta-1-alpha (Avonex)), glatiramer acetate (Copaxone), teriflunomide (Aubagio), dimethyl fumarate (Tecfidera) and ocrelizumab (Ocrevus).
There are approximately 1700 patients currently on funded multiple sclerosis treatments. There are funding criteria in place for these treatments and patients’ eligibility for funded treatment is currently determined by a panel of clinicians contracted by PHARMAC, the Multiple Sclerosis Treatment Assessment Committee (MSTAC).
- Natalizumab, teriflunomide, dimethyl fumarate, ocrelizumab, and fingolimod are currently funded from first confirmed diagnosis of definite relapsing remitting MS, for patients with an EDSS score of 0 to 4.0, who meet the funding criteria. The Kurtzke Expanded Disability Status Scale (EDSS) is a method of quantifying disability in multiple sclerosis and is used to measure and assess disability and disease progression in MS.
- The beta interferons and glatiramer acetate are currently funded for those patients who cannot take fingolimod or natalizumab for clinical reasons, and for patients who had funding approvals issued before 1 November 2014.
Why we’re proposing this
Currently, members of PHARMAC’s MSTAC assess whether applications meet the eligibility criteria for accessing funded multiple sclerosis treatments. The current funding criteria target funding for multiple sclerosis treatments to patients with clinically definite relapsing remitting multiple sclerosis (CD RR-MS) with an EDSS from 0 to 4.0. Our proposal is to widen access to patients with an EDSS score of 0 to 6.0 (inclusive) which would allow most patients to stay on treatment for longer. An EDSS of 6.0 means that a person can walk 100m with or without rest and/or assistance (including a cane, crutch or brace).
Transitioning access to multiple sclerosis treatments to a standard Special Authority would provide a more streamlined and faster mechanism for clinicians applying for funded multiple sclerosis treatments for their patients. The current process is time-consuming as it involves the completion of a multi-page application form, submission to PHARMAC and a delay in the issuing of approval numbers by up to a month (as MSTAC only meet every three-four weeks).
If the electronic Special Authority system is used the clinician would receive the approval number at the same time as the patient’s EDSS is assessed. The patient would then have an approval number and prescription at the time of the assessment, rather than having to wait for up to a month for the approval.
PHARMAC has sought advice from MSTAC, the Pharmacology and Therapeutics Advisory Committee (PTAC) and the Neurological Subcommittee of PTAC on an application to amend the stopping criteria for all funded multiple sclerosis treatments.
We also sought advice from MSTAC and the Neurological Subcommittee of PTAC to develop appropriate Special Authority criteria to enable a transition from Panel assessment to standard Special Authority. The clinical advisors recommended transferring funded access to multiple sclerosis treatments to a standard Special Authority that would contain revised, but similar, criteria. Considerations in relation to the transition from Panel to Special Authority included:
- ensuring patients who started treatment under older eligibility criteria are not disadvantaged by this change.
- generating one standard set of Special Authority criteria for all currently funded multiple sclerosis treatments.
- removing criteria that are overly prescriptive and leaving certain clinical decisions to be made by clinicians acting within their scope of practice.
This proposal to transition to a standard Special Authority and widen funded access to multiple sclerosis treatments is in line with the recommendations from our clinical advisors and would allow most patients to stay on a funded treatment for longer.
Details about our proposal
From 1 March 2021 all funded multiple sclerosis treatments would be subject to the Special Authority criteria outlined below.
The Special Authority would apply for each and all of the currently funded MS treatments (ie. interferon beta-1-alpha, interferon beta-1-beta, glatiramer acetate, fingolimod, dimethyl fumarate, natalizumab, teriflunomide, and ocrelizumab) combined, as one single set of criteria (rather than as separate sets of criteria for each MS treatment, as occurs currently). This would allow clinicians to switch their patient’s treatment to a different chemical if clinically appropriate, without needing to apply for a new initial Special Authority approval.
Changes from the current access criteria are shown in bold (for additions) and strikethrough (for deletions).
Initial application – (Multiple sclerosis) from a neurologist or general physician.
Approvals valid for 12 months meeting the following criteria:
Entry Criteria All of the following
- Diagnosis of multiple sclerosis (MS) must be confirmed by a neurologist. Diagnosis must include MRI confirmation; and
- Patients must have Clinically Definite Relapsing Remitting MS with or without underlying progression; and
- Patients must have an EDSS score between 0 - 4.0 0 – 6.0; and
- Both:
- Patient has had at least 1 significant relapse of MS in the previous 12 months or 2 significant relapses in the past 24 months; and
- All of the following:
- Each significant relapse must be confirmed by the applying neurologist or general physician (the patient may not necessarily have been seen by them during the relapse but the neurologist/physician must be satisfied that the clinical features were characteristic); and
- Each significant relapse is associated with characteristic new symptom(s)/sign(s) or substantially worsening of previously experienced symptoms(s)/sign(s); and
- Each significant relapse has lasted at least one week; and
- Each significant relapse has started at least one month after the onset of a previous relapse; and
- Either
- Each significant relapse is severe enough to change either the EDSS or at least one of the Kurtze Functional System scores by at least 1 point; or
- Each significant relapse is a recurrent paroxysmal symptom of MS (tonic seizures/spasms, trigeminal neuralgia, Lhermitte’s symptom); and
- Each significant relapse can be distinguished from the effects of general fatigue; and is not associated with a fever (T> 37.5°C); and
- Both
- Evidence of new inflammatory activity on an MR scan within the past 24 months; and
- Any of the following:
- Sign of that new inflammatory activity is a gadolinium enhancing lesion; or
- Sign of that new inflammatory activity is a lesion showing diffusion restriction; or
- Sign of that new inflammatory is a T2 lesion with associated local swelling; or
- Sign of that new inflammatory activity is a prominent T2 lesion that clearly is responsible for the clinical features of a recent relapse that occurred within the last 2 years; or
- Sign of that new inflammatory activity is new T2 lesions compared with a previous MR scan.
-
- Applications must be made by the patient's neurologist or general physician; and.
- Patients must have no previous history of lack of response to [for each individual MS treatment’s Special Authority criteria, state here the relevant newer MS treatment (natalizumab, ocrelizumab, fingolimod, teriflunomide or dimethyl fumarate as relevant)]; and
- Patients must have not previously had intolerance to [for each individual MS treatment’s Special Authority criteria, state here the relevant newer MS treatment (natalizumab, ocrelizumab, fingolimod, teriflunomide or dimethyl fumarate as relevant)].; and
- Patient must not be co-prescribed beta interferon or glatiramer acetate.
- Applications must be made by the patient's neurologist or general physician; and.
Note: Natalizumab can only be dispensed from a pharmacy registered in the Tysabri Australasian Prescribing Programme operated by the supplier. Switching between natalizumab, fingolimod, dimethyl fumarate, teriflunomide and ocrelizumab is permitted provided the EDSS stopping criteria are not met. Switching to interferon or glatiramer acetate is only permitted provided the EDSS stopping criteria are not met and both fingolimod and natalizumab are either not tolerated or treatment with both agents would be clinically inappropriate. Continued relapses on treatment would be expected to lead to a switch of treatment provided the stopping criteria are not met. If a relapse has resulted in an increased EDSS score that potentially may lead to discontinuation of treatment according to stopping criteria, a period of 6 months is allowed from the start of the relapse for recovery to occur. Treatment on two funded multiple sclerosis treatments simultaneously is not permitted.
Renewal application – (Multiple sclerosis) only from a neurologist or general physician.
Approvals valid for 12 months where patient has had an EDSS score of 0 to 6.0 at any time in the last six months (i.e. the patient has walked 100 metres or more with or without aids in the last six months).
Stopping criteria
Any of the following:
- Confirmed progression of disability that is sustained for six months during a minimum of one year of treatment. Progression of disability is defined as progress by any of the following EDSS Points:
- From starting at EDSS 0 increasing to (i.e. stopping on reaching) EDSS 3.0; or;
- 0 to 3.0; or
- 5 to 3.5; or
- 0 to 4.0; or
- 5 to 4.5; or
- 0 to 4.5; or
- 0 to 4.5.
- Increasing relapse rate over 12 months of treatment (compared with relapse rate on starting treatment) (see note); or and
- Intolerance to treatment; or
- Non-compliance with treatment, including refusal to undergo annual assessment
- Patients must have either:
- 5.1 Intolerance to both natalizumab and fingolimod; or
- 5.2 Treatment with both natalizumab and fingolimod is considered clinically inappropriate.
Note: Natalizumab can only be dispensed from a pharmacy registered in the Tysabri Australasian Prescribing Programme operated by the supplier. Switching between natalizumab, fingolimod, dimethyl fumarate, teriflunomide and ocrelizumab is permitted provided the EDSS stopping criteria are not met. Switching to interferon or glatiramer acetate is only permitted provided the EDSS stopping criteria are not met. Switching to interferon or glatiramer acetate is only permitted provided the EDSS criteria are not met and both fingolimod and natalizumab are either not tolerated or treatment with both agents would be clinically inappropriate. Continued relapses on treatment would be expected to lead to a switch of treatment provided the stopping criteria are not met. If a relapse has resulted in an increased EDSS score that potentially may lead to discontinuation of treatment according to stopping criteria, a period of 6 months is allowed from the start of the relapse for recovery to occur. Treatment on two funded multiple sclerosis treatments simultaneously is not permitted.
Schematically, these changes can be depicted in the following diagram:
All current initial and renewal MSTAC approvals would be converted to new Special Authority approvals, to ensure funded access to treatment continues for these patients.
Patients with current approvals under the older eligibility criteria that allow them to stay on treatment above EDSS of 6.0 would be ‘grand-parented’ and would be granted approval numbers through the Exceptional Circumstances process to ensure that they would be able to remain on their current treatments with their current stopping criteria. This applies to 10 patients. We would communicate directly with the clinicians of these patients to inform them of the process to apply for an annual renewal.
To provide feedback
Send us an email: mstaccoordinator@pharmac.govt.nz by 5pm Monday, 21 December 2020.
Your feedback may be shared
Feedback we receive is subject to the Official Information Act 1982 (OIA). Please be aware that we may need to share your feedback, including your identity, in response to an OIA request. This applies to anyone providing feedback, whether they are providing feedback themselves or for an organisation, in a personal or professional capacity.
We can only keep feedback confidential as allowed under the OIA and other related laws. If you want any part of your feedback treated as confidential, you need to tell us. Please let us know if you want to keep part of your feedback confidential, and why. Is it commercially sensitive, confidential or proprietary, or personal information? Clearly state this and tell us which parts of your feedback you want to keep confidential for these reasons. We will consider your request under our OIA requirements.