Decision to widen access to secukinumab for psoriatic arthritis and ankylosing spondylitis
What we’re doing
We are pleased to announce a decision to widen funded access to secukinumab (Cosentyx) for the treatment of psoriatic arthritis or ankylosing spondylitis, through an agreement with Novartis New Zealand Limited (Novartis), from 1 May 2021.
This decision will result in the following changes:
- secukinumab will be funded as a first line biologic for psoriatic arthritis
- secukinumab will be funded as a second line biologic for ankylosing spondylitis
- a new secukinumab pack size will be listed, enabling the dispensing of singular packs.
We estimate that approximately 200 patients will benefit in the first year. After five years, we estimate that up to 1,000 patients will benefit each year.
The agreement with Novartis also includes amendments to the contractual arrangements for fingolimod capsules (Gilenya) and ciclosporin capsules and oral liquid (Neoral) but there are no changes to the funding of these products. More details are provided below.
Any changes to the original proposal?
This decision was subject to a consultation letter dated 5 February 2021. Following consideration of the feedback received, we have made minor changes to the Special Authority criteria for adalimumab, etanercept and infliximab to ensure that people can transition between treatments if they experience intolerable side effects or do not receive an adequate response to secukinumab.
You can find the consultation letter on the PHARMAC website here.
Consultation is an important part of our process and we are grateful to all those who provided consultation feedback. The consultation responses we received and our response to the points raised are summarised below.
Who we think will be most interested
- People with psoriatic arthritis or ankylosing spondylitis and their whānau, family, and caregivers
- Rheumatologists, specialist nurses, general practitioners, dermatologists, and other health professionals involved in the care of people with psoriatic arthritis and ankylosing spondylitis
- Hospital and community pharmacists, DHBs and wholesalers
- Pharmaceutical suppliers
- Other organisations with an interest in rheumatological conditions and treatments
Detail about this decision
The following changes will occur in Section B and Part II of Section H of the Pharmaceutical Schedule.
Secukinumab
Funded access to secukinumab will be amended in Section B and Part II of Section H of the Pharmaceutical Schedule from 1 May 2021 to include people with psoriatic arthritis or ankylosing spondylitis as follows (new criteria shown only):
Special Authority for Subsidy
Initial application — (psoriatic arthritis) only from a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria:
Either:
- Both:
- The patient has had an initial Special Authority approval for adalimumab or etanercept for psoriatic arthritis; and
- Either:
- The patient has experienced intolerable side effects from adalimumab or etanercept; or
- The patient has received insufficient benefit from adalimumab or etanercept to meet the renewal criteria for adalimumab or etanercept for psoriatic arthritis; or
- All of the following:
- Patient has had severe active psoriatic arthritis for six months duration or longer; and
- Patient has tried and not responded to at least three months of oral or parenteral methotrexate at a dose of at least 20 mg weekly or a maximum tolerated dose; and
- Patient has tried and not responded to at least three months of sulfasalazine at a dose of at least 2 g per day or leflunomide at a dose of up to 20 mg daily (or maximum tolerated doses); and
- Either:
- Patient has persistent symptoms of poorly controlled and active disease in at least 15 swollen, tender joints; or
- Patient has persistent symptoms of poorly controlled and active disease in at least four joints from the following: wrist, elbow, knee, ankle, and either shoulder or hip; and
- Any of the following:
- Patient has a C-reactive protein level greater than 15 mg/L measured no more than one month prior to the date of this application; or
- Patient has an elevated erythrocyte sedimentation rate (ESR) greater than 25 mm per hour; or
- ESR and CRP not measured as patient is currently receiving prednisone therapy at a dose of greater than 5 mg per day and has done so for more than three months.
Renewal — (psoriatic arthritis) only from a rheumatologist or practitioner on the recommendation of a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria:
Both:
- Either:
- Following 3 to 4 months' initial treatment, the patient has at least a 50% decrease in active joint count from baseline and a clinically significant response to treatment in the opinion of the physician; or
- The patient demonstrates at least a continuing 30% improvement in active joint count from baseline and a clinically significant response to prior secukinumab treatment in the opinion of the treating physician; and
- Secukinumab to be administered at doses no greater than 300 mg monthly.
Initial application — (ankylosing spondylitis – second-line biologic) only from a rheumatologist or practitioner on the recommendation of a rheumatologist. Approvals valid for 3 months for applications meeting the following criteria:
Both:
- The patient has had an initial Special Authority approval for adalimumab and/or etanercept for ankylosing spondylitis; and
- Either
- The patient has experienced intolerable side effects from a reasonable trial of adalimumab and/or etanercept; or
- Following 12 weeks of adalimumab and/or etanercept treatment, the patient did not meet the renewal criteria for adalimumab and/or etanercept for ankylosing spondylitis.
Renewal — (ankylosing spondylitis – second-line biologic) only from a rheumatologist or practitioner on the recommendation of a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria:
All of the following:
- Following 12 weeks initial treatment of secukinumab treatment, BASDAI has improved by 4 or more points from pre-secukinumab baseline on a 10 point scale, or by 50%, whichever is less; and
- Physician considers that the patient has benefitted from treatment and that continued treatment is appropriate; and
- Secukinumab to be administered at doses no greater than 150 mg monthly.
Similar restrictions will apply to Part II of Section H of the Pharmaceutical Schedule.
There are no changes to other existing Special Authority criteria or hospital restrictions for secukinumab.
Secukinumab inj 150 mg per ml, 1 ml prefilled syringe (1 prefilled syringe pack) will be listed in Section B and Part II of Section H of the Pharmaceutical Schedule, subject to the funding restrictions above, from 1 May 2021 at the following price and subsidy (ex-manufacturer, excluding GST):
|
Chemical |
Formulation |
Brand |
Pack |
Price and |
|---|---|---|---|---|
|
Secukinumab |
Inj 150 mg per ml, 1 ml prefilled syringe |
Cosentyx |
1 |
$799.50 |
This will allow dispensing of singular packs of secukinumab.
A confidential rebate will apply to Novartis’ brand of secukinumab (Cosentyx) that reduces the net price to the Funder. It will have protection from delisting and subsidy reduction until 30 April 2023. The list price of Cosentyx, two prefilled syringe pack will not change as a result of this decision.
Adalimumab, etanercept and infliximab
The Special Authority criteria for adalimumab, etanercept and infliximab for psoriatic arthritis will be amended to allow patients to move between first-line and second-line biologic treatments.
This means that people will be able to access funded secukinumab after trialling adalimumab or etanercept (and vice-versa) if they experience intolerable side effects or do not receive adequate benefit. It will also allow patients to access funded infliximab after trialling secukinumab.
The amendments are as follows (adalimumab shown only):
Initial application — (psoriatic arthritis) only from a rheumatologist. Approvals valid for 6 months for applications meeting the following criteria:
Either:
- Both:
- The patient has had an initial Special Authority approval for etanercept or secukinumab for psoriatic arthritis; and
- Either:
- The patient has experienced intolerable side effects from etanercept or secukinumab; or
- The patient has received insufficient benefit from etanercept or secukinumab to meet the renewal criteria for etanercept or secukinumab for psoriatic arthritis; or
- All of the following:
- Patient has had severe active psoriatic arthritis for six months duration or longer; and
- Patient has tried and not responded to at least three months of oral or parenteral methotrexate at a dose of at least 20 mg weekly or a maximum tolerated dose; and
- Patient has tried and not responded to at least three months of sulfasalazine at a dose of at least 2 g per day or leflunomide at a dose of up to 20 mg daily (or maximum tolerated doses); and
- Either:
- Patient has persistent symptoms of poorly controlled and active disease in at least 15 swollen, tender joints; or
- Patient has persistent symptoms of poorly controlled and active disease in at least four joints from the following: wrist, elbow, knee, ankle, and either shoulder or hip; and
- Any of the following:
- Patient has a C-reactive protein level greater than 15 mg/L measured no more than one month prior to the date of this application; or
- Patient has an elevated erythrocyte sedimentation rate (ESR) greater than 25 mm per hour; or
- ESR and CRP not measured as patient is currently receiving prednisone therapy at a dose of greater than 5 mg per day and has done so for more than three months.
Similar additions have been made to the Special Authority criteria for etanercept and infliximab. There are no changes to other existing Special Authority criteria or hospital restrictions for adalimumab, etanercept or infliximab.
The following diagram depicts the treatment pathway for patients requiring treatments for psoriatic arthritis. Patients usually move within each group of treatments before progressing to the next group of medicines.
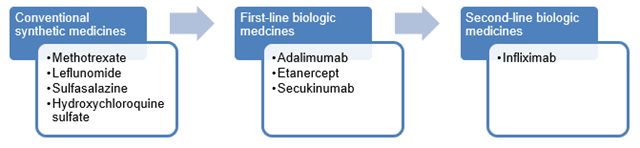
Fingolimod and ciclosporin
Novartis’ brand of fingolimod capsules (Gilenya) and ciclosporin capsules and oral liquid (Neoral) will have protection from delisting and subsidy reduction until 31 December 2022. The net price of these products will also reduce as a result of a new confidential rebate.
The list price of Novartis’ brand of fingolimod and ciclosporin (Gilenya and Neoral) will not change as a result of this decision. There are no other changes to the listings of fingolimod and ciclosporin.
Our response to what you told us
We are grateful for the time people took to respond to this consultation. A summary of the main themes raised in feedback, our responses to the feedback received, and changes we have made after listening to you are available on our notification webpage.
|
Theme |
PHARMAC Comment |
|---|---|
|
Responders were generally supportive of the proposal |
We are really grateful to everyone who took the time to provide feedback and share their personal stories with us. We believe this decision will result in positive outcomes for patients |
|
Requests to change the Special Authority criteria for adalimumab and etanercept for psoriatic arthritis to allow people who have experienced intolerable side effects or received insufficient benefit from secukinumab for psoriatic arthritis to transition to an alternative funded biologic treatment |
Following consideration of the feedback received, we have also amended the Special Authority criteria for adalimumab, etanercept and infliximab for psoriatic arthritis, as detailed above |
|
Concern regarding ability to access funded secukinumab where people self-funding secukinumab may not meet the proposed criteria |
We are happy to consider funding for patients who have accessed treatments through a different funding pathway (eg moving from overseas, self-funding, clinical trials) via the Special Authority Waiver process. Prescribers can find out how to apply for Special Authority waivers on the PHARMAC website |
|
Requests to change or remove the requirement for abnormal CRP, ESR or regular prednisone administration |
The Special Authority criteria align with that of currently funded psoriatic arthritis biologic treatments. We will seek advice from our clinical advisors regarding amending the criteria |
|
Requests to change the proposed psoriatic arthritis Special Authority criteria to reflect current international guidelines, which suggest secukinumab should be a second-line biologic treatment, unless there is significant skin involvement |
Our clinical advisors recommended secukinumab as a first-line biologic treatment for psoriatic arthritis. The Special Authority criteria allow clinicians to choose the best first-line biologic treatment option for their patients |
If you have any questions about this decision, you can email us at enquiry@pharmac.govt.nz; or call our toll free number (9 am to 5 pm, Monday to Friday) on 0800 660 050.


